Cation And Anion Chart
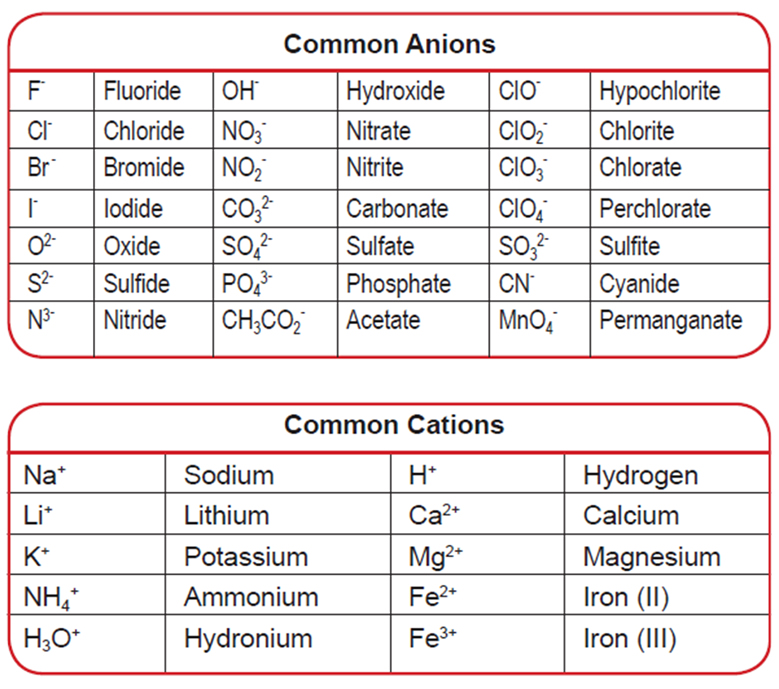
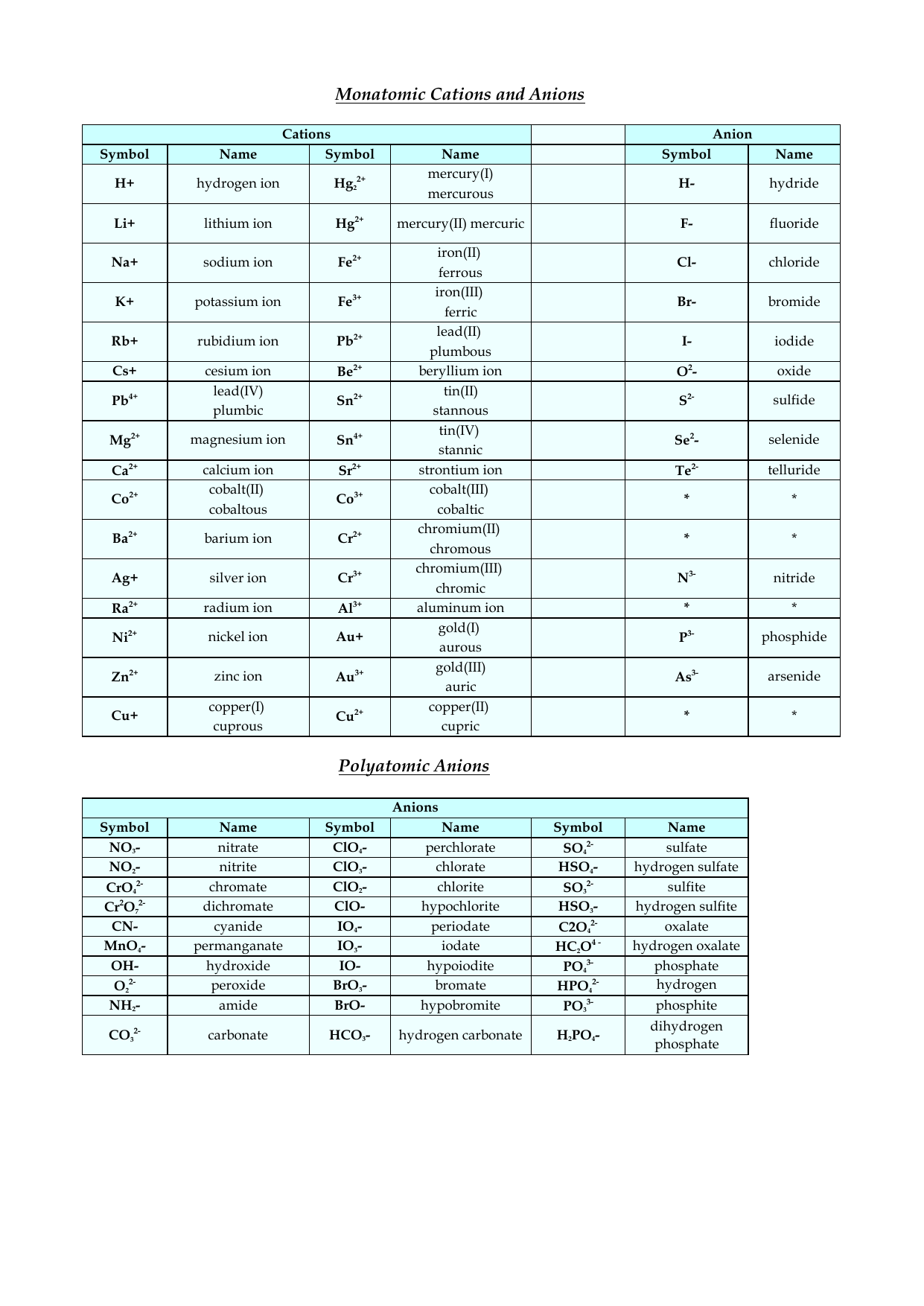
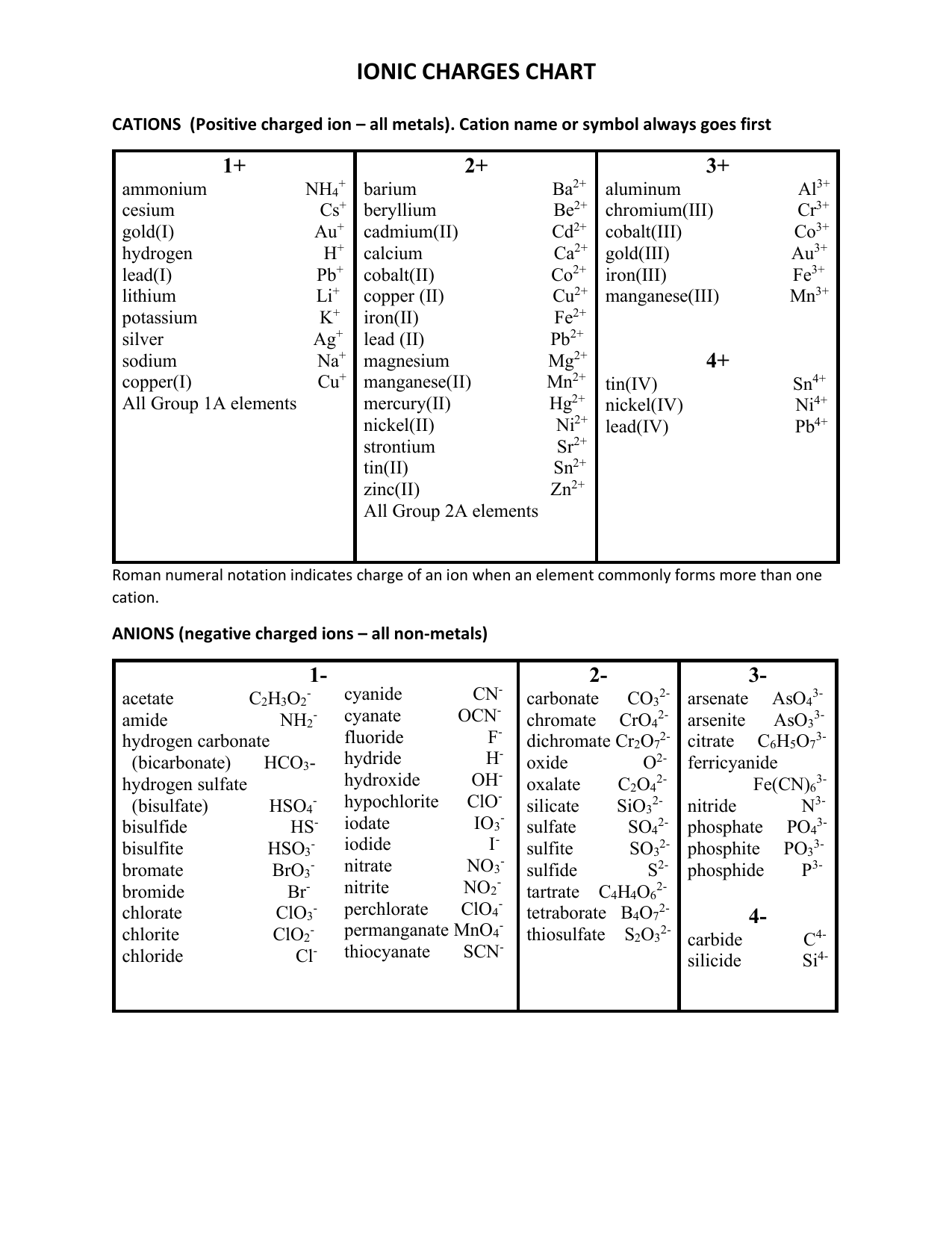
Cation And Anion Chart - Ions are charged atoms or molecules. Web cations and anions are the two types of ions. Web cations are ions that are positively charged. What is the difference between cations and anions in terms of reactivity? Web salts (made of ions, neutral in charge) name: (cation) m(anion) n·(#)h 2o (if # = 0 h 2o is omitted) examples cations on the left, anions on the right, charge must cancel strontium chloride = strontium ions and chloride ions = sr+2 and cl‐ = srcl 2 Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the periodic table. Web when h + is written in equations or textbooks, it usually is a simplified way of saying h3o+. Anions are ions that are negatively charged. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. If a balanced atom loses one or more electrons, it will become a positively charged cation. Anions have a negative electrical charge and have more electrons than protons. Web when h + is written in equations or textbooks, it usually is a simplified way of saying h3o+. Web cations are positively charged ions formed when neutral atoms lose electrons; Web. Ions are charged atoms or molecules. Web cations and anions are the two types of ions. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the periodic table. Web cations are positively charged ions formed when neutral atoms lose. Anions have a negative electrical charge and have more electrons than protons. Anions are grouped according to whether they are simple (one element or monatomic ions ), oxoanions (containing oxygen), derived from organic acids, or. Web cations are positively charged ions formed when neutral atoms lose electrons; Web in this tutorial, you will learn about the properties, differences, and examples. Anions have a negative electrical charge and have more electrons than protons. If a balanced atom gains one or more electrons, it will become a negatively charged anion. If a balanced atom loses one or more electrons, it will become a positively charged cation. It is possible to predict the charges of main group ions by looking at the group. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. (cation) m(anion) n·(#)h 2o (if # = 0 h 2o is omitted) examples cations on the left, anions on the right, charge must cancel strontium chloride = strontium ions and chloride ions = sr+2 and cl‐ = srcl 2 Ions are charged atoms or. Mercury (i) ion exists as a diatomic unit. Anions are ions that are negatively charged. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the periodic table. Ions have an imbalance of electrical charge, meaning they contain different numbers. Web the ion names, formulas and charges chart for chemistry classrooms comprehensively lists the names and formulas of cations and anions, allowing students to quickly determine formulas for various combinations of cations and anions. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Anions are grouped according to whether they are simple (one. Anions are ions that are negatively charged. Web salts (made of ions, neutral in charge) name: Ions are charged atoms or molecules. Web when h + is written in equations or textbooks, it usually is a simplified way of saying h3o+. What is the difference between cations and anions in terms of reactivity? Mercury (i) ion exists as a diatomic unit. What is the difference between cations and anions in terms of reactivity? Anions are negatively charged ions formed when neutral atoms gain electrons. Ions are charged atoms or molecules. Web cations are ions that are positively charged. Web when h + is written in equations or textbooks, it usually is a simplified way of saying h3o+. Anions are negatively charged ions formed when neutral atoms gain electrons. Web cations are positively charged ions formed when neutral atoms lose electrons; Web cations are ions that are positively charged. It is possible to predict the charges of main group. Anions are negatively charged ions formed when neutral atoms gain electrons. If a balanced atom loses one or more electrons, it will become a positively charged cation. Web the ion names, formulas and charges chart for chemistry classrooms comprehensively lists the names and formulas of cations and anions, allowing students to quickly determine formulas for various combinations of cations and anions. Anions are ions that are negatively charged. What is the difference between cations and anions in terms of reactivity? Cation have a positive electrical charge and have more protons than electrons. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Web this table lists common anions by their names, with the anion formula and charge. Mercury (i) ion exists as a diatomic unit. Web in this tutorial, you will learn about the properties, differences, and examples of ions, cations and anions, as well as how to predict them based on their positions on the periodic table. It is possible to predict the charges of main group ions by looking at the group numbers on the periodic table. Web cations are positively charged ions formed when neutral atoms lose electrons; (cation) m(anion) n·(#)h 2o (if # = 0 h 2o is omitted) examples cations on the left, anions on the right, charge must cancel strontium chloride = strontium ions and chloride ions = sr+2 and cl‐ = srcl 2 Ions are charged atoms or molecules. Web salts (made of ions, neutral in charge) name: Web cations and anions are the two types of ions.
Cations and Anions Definitions, Examples, and Differences
Periodic Table List Of Cations And Anions Periodic Table Timeline
List of Cations and Anions PDF Ion Hydrogen
ListofCationsandAnions (1)
Cations And Anions Chart
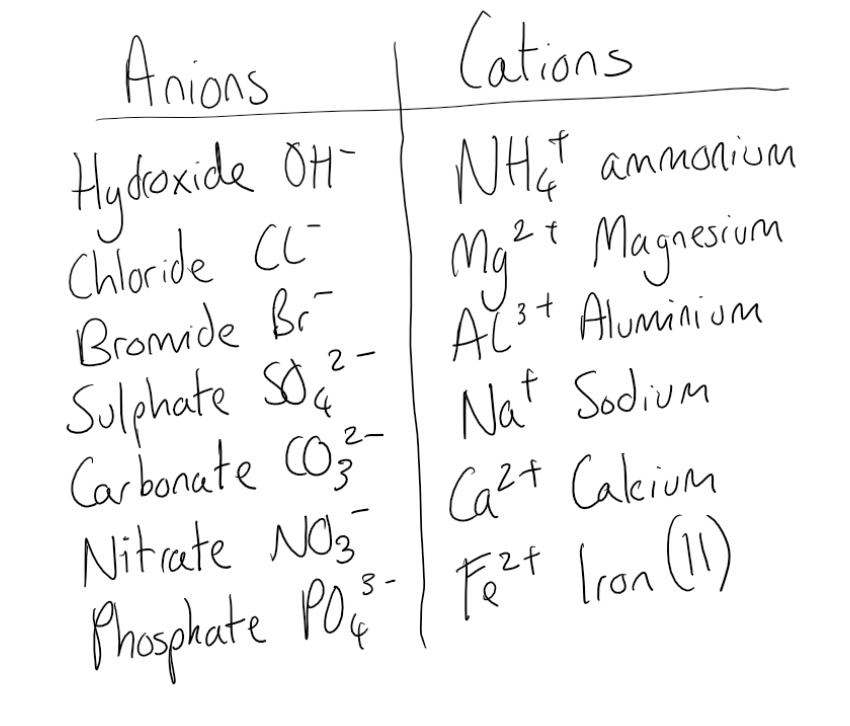
Anion Cation Common Names Formulas And Charges For Familiar Ions Ions

Cations And Anions Chart

Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry
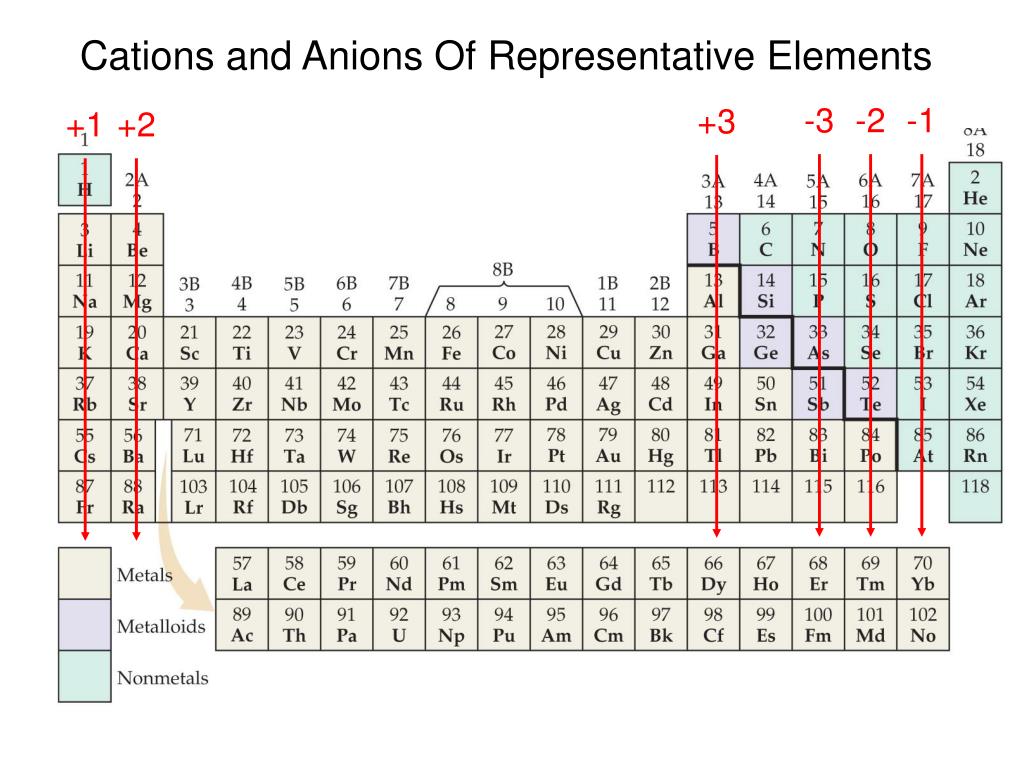
PPT Mastering Chemistry PowerPoint Presentation, free download ID
Common Ions Anions and Cations PDF
Anions Have A Negative Electrical Charge And Have More Electrons Than Protons.
Web When H + Is Written In Equations Or Textbooks, It Usually Is A Simplified Way Of Saying H3O+.
Anions Are Grouped According To Whether They Are Simple (One Element Or Monatomic Ions ), Oxoanions (Containing Oxygen), Derived From Organic Acids, Or.
(Cation)(Anion) (Prefix)Hydrate (If # = 0 Hydrate Is Omitted) Formula:
Related Post:

