Case Report Form Template
Case Report Form Template - It is commonly used in the medical field. Web case report form template. Importance of pilot testing, change management and optimization of data architecture. Its development represents a significant part of the clinical trial and can affect study success. A case report form is used to document an injury or illness. Sample clinical trial closeout procedures. The institute for clinical and translational research is supported by the clinical and translational science award (ctsa) program, the national center for advancing translational sciences (ncats), grant 1ul1tr002373. Web 4240 health sciences learning center. Informed consent and counselling : Information pertaining to a patient’s illness or injury is recorded in the blue boxes. Importance of pilot testing, change management and optimization of data architecture. Prior and concomitant medications form. A final crf is generally not a single form, but a series of individual case report forms that gather data related to the trial. Web interviewer follow up form : Capture, analyze, and organize patient data with unprecedented ease and accuracy. A final crf is generally not a single form, but a series of individual case report forms that gather data related to the trial. Prior and concomitant medications form. In some cases, the medical report may be used to document difficulty in diagnosing a patient’s ailment or condition. The subject is male or female, between 25 and 65 years of. What is the main message of your case report on the basis of this “core”? The institute for clinical and translational research is supported by the clinical and translational science award (ctsa) program, the national center for advancing translational sciences (ncats), grant 1ul1tr002373. In some cases, the medical report may be used to document difficulty in diagnosing a patient’s ailment. While paper crfs require physical storage, security, and transportation, collecting clinical data in a digital format. 750 highland avenue madison, wi 53705. Planning how and what data to collect in an investigator initiated trial (iit) is one of the most challenging tasks for researchers. With this case report form, sponsors and medical practitioners collect the specific data they need in. Some reports contain an extensive review of the. Now you need to write your background, discussion, and conclusion sections. Web interviewer follow up form : While paper crfs require physical storage, security, and transportation, collecting clinical data in a digital format. The necessity of incorporating data sharing requirements. Its development represents a significant part of the clinical trial and can affect study success. Web free 20+ case report form samples in pdf. Establishing a library of case report form templates expedites the design of individual crfs and ensures consistency in data collection. The institute for clinical and translational research is supported by the clinical and translational science award. A case report form is used to document an injury or illness. Importance of pilot testing, change management and optimization of data architecture. Web case report form template. Sample clinical trial closeout procedures. A final crf is generally not a single form, but a series of individual case report forms that gather data related to the trial. A case report form is used to document an injury or illness. Sample clinical trial closeout procedures. What is the main message of your case report on the basis of this “core”? Capture, analyze, and organize patient data with unprecedented ease and accuracy. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation. Every one can create a case report form (crf)! Read our author guide for more information. Web how to prepare case report forms. A case report form is used to document an injury or illness. Web 4240 health sciences learning center. In some cases, the medical report may be used to document difficulty in diagnosing a patient’s ailment or condition. If you are looking to increase study start up timelines, decrease overall trial cost, and further reduce the time burden of manual processes, request access to our clinical trial optimization kit resources below. [ 1] site personnel capture the subject's data. A case report form is used to document an injury or illness. Audiovisual recording of informed consent sop. Some reports contain an extensive review of the. It supports healthcare professionals and researchers by streamlining data collection and analysis. With these seven steps, you have the “core” of your case report. Informed consent and counselling : [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Reviewing and obtaining informed consent sop. A final crf is generally not a single form, but a series of individual case report forms that gather data related to the trial. With this case report form, sponsors and medical practitioners collect the specific data they need in order to test their hypotheses or answer their research questions. The necessity of incorporating data sharing requirements. Now you need to write your background, discussion, and conclusion sections. Web free case report form template. Web 4240 health sciences learning center. Web an electronic case report form is an online questionnaire used for data collection in medical studies and clinical trials. Key design elements and inclusion of standard measures.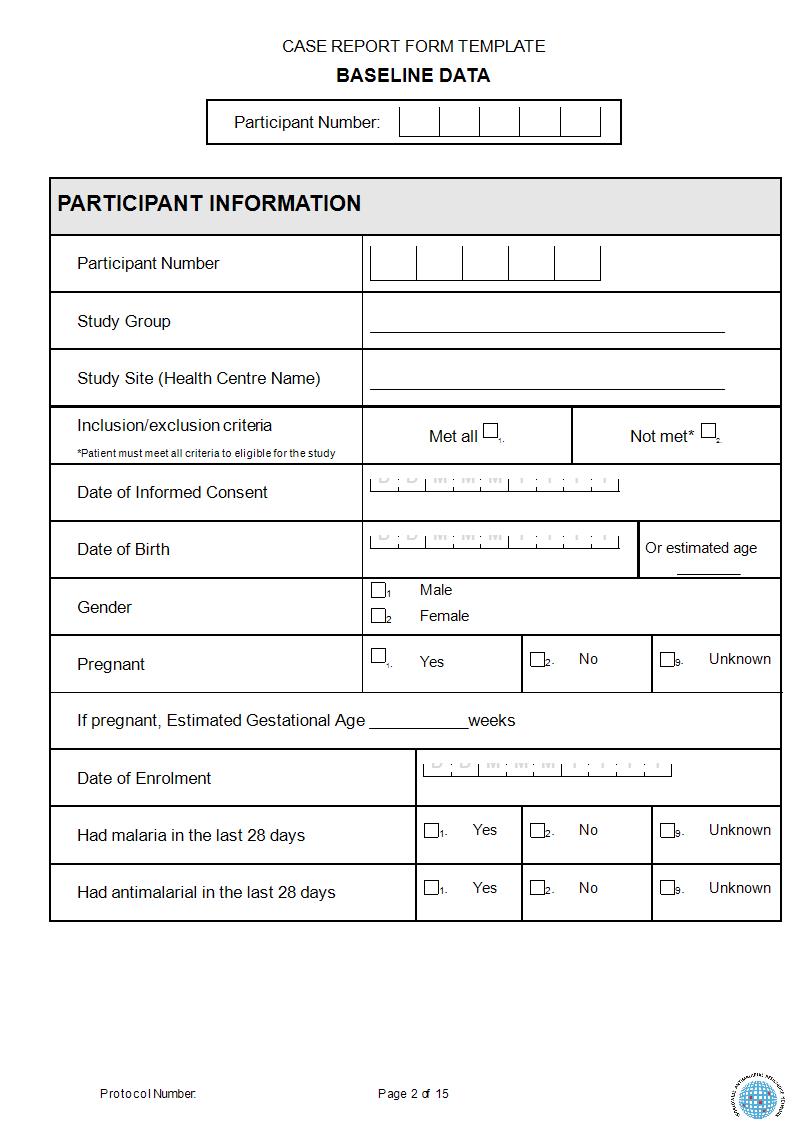
FREE 15+ Case Report Forms in PDF MS Word

FREE 15+ Case Report Forms in PDF MS Word

FREE 15+ Case Report Forms in PDF MS Word
FREE 15+ Case Report Forms in PDF MS Word

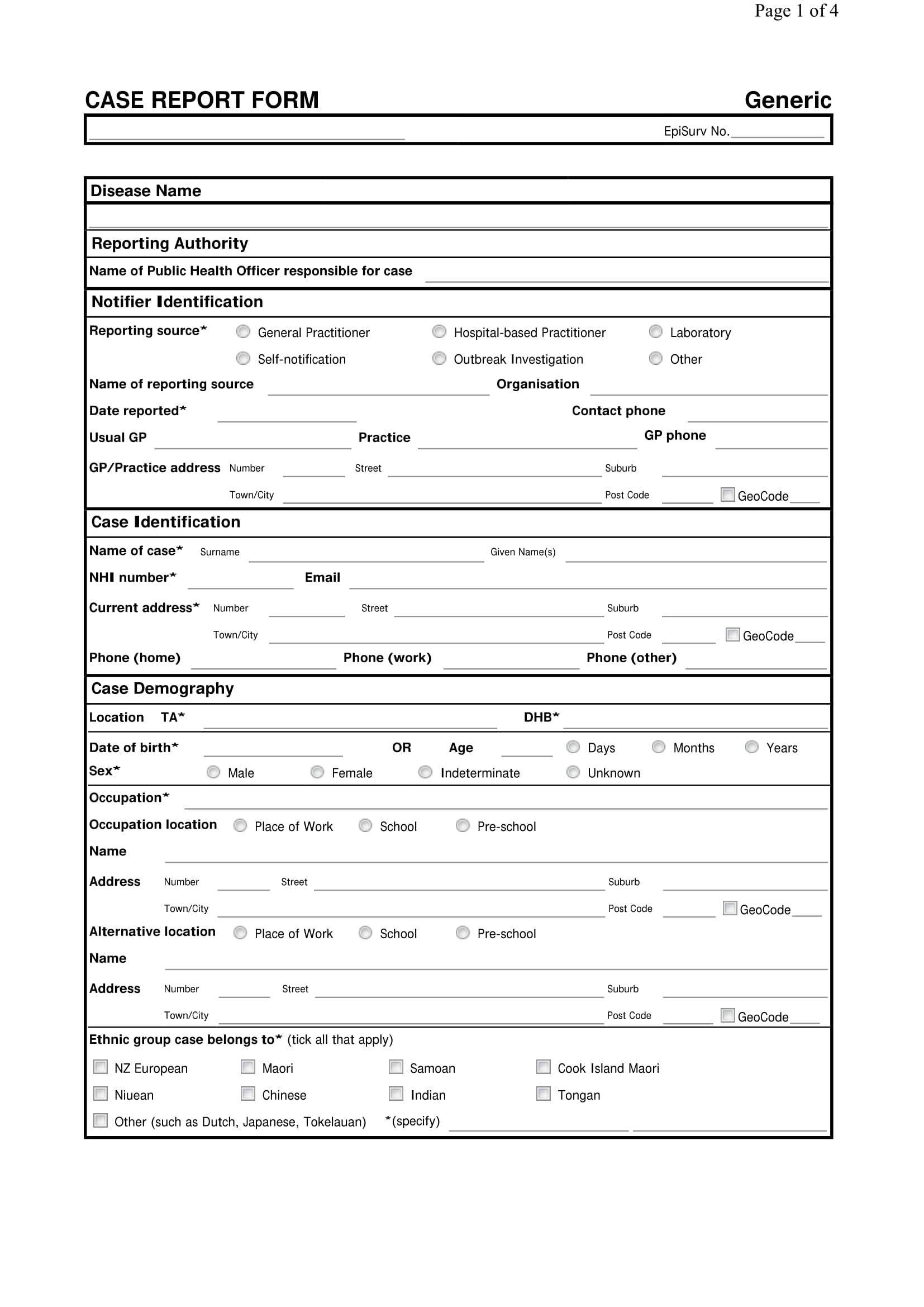
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs
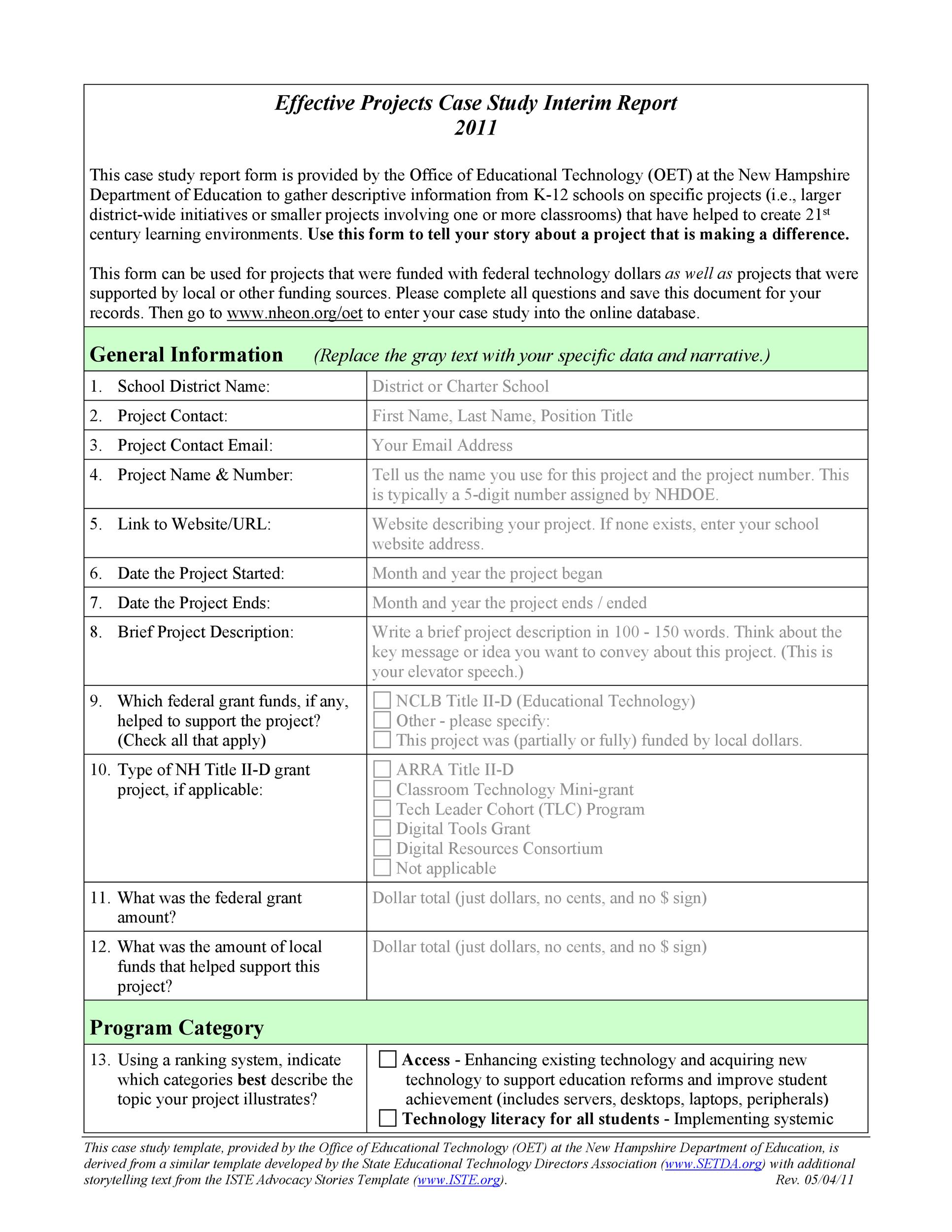
Case Report Form Template Sampletemplate.my.id
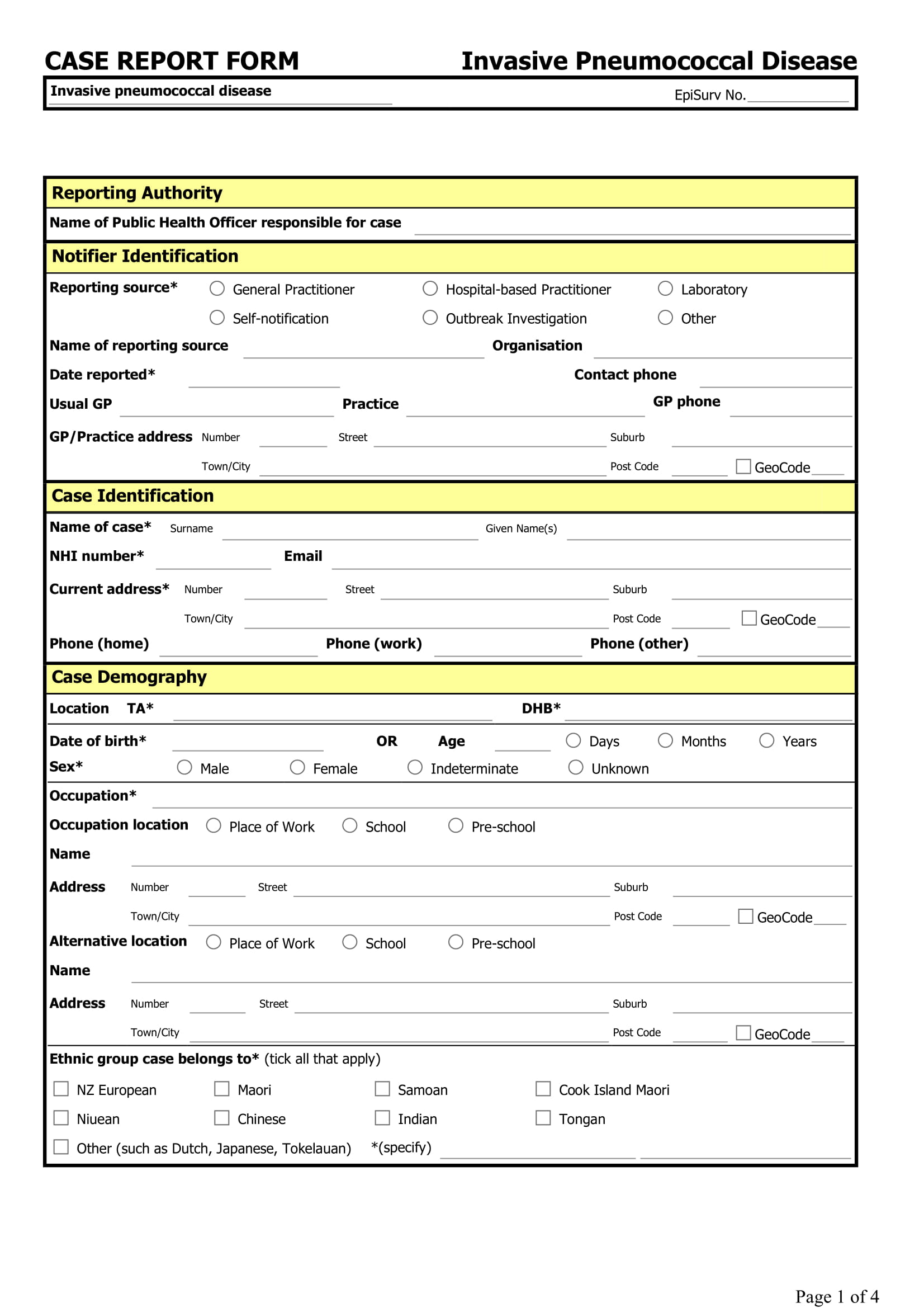
FREE 15+ Case Report Forms in PDF MS Word

FREE 15+ Case Report Forms in PDF MS Word

FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs
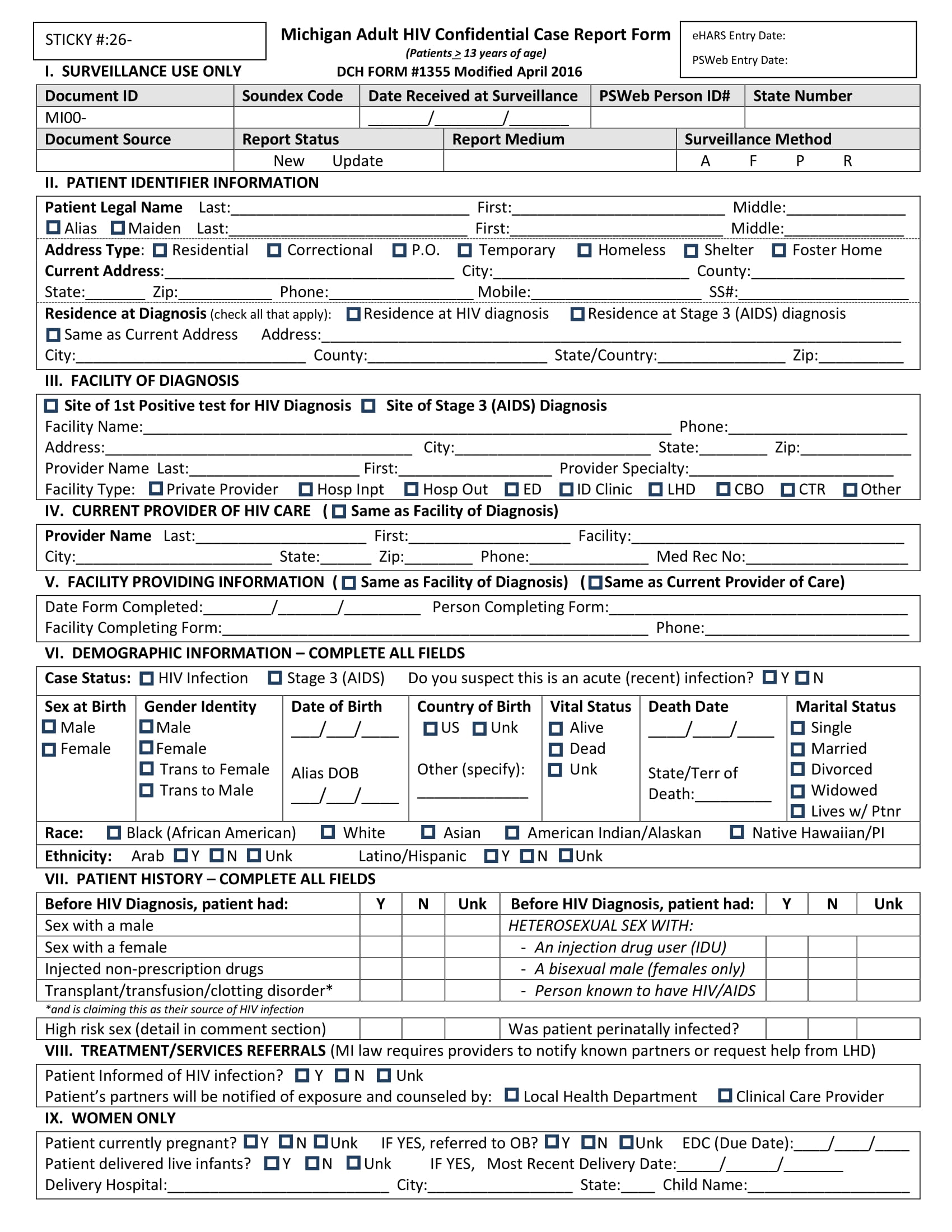
FREE 15+ Case Report Forms in PDF MS Word
Web Interviewer Follow Up Form :
Electronic Case Report Forms Support More Accurate Data Collection And Faster Data.
The Institute For Clinical And Translational Research Is Supported By The Clinical And Translational Science Award (Ctsa) Program, The National Center For Advancing Translational Sciences (Ncats), Grant 1Ul1Tr002373.
The Initial Section Of The Form Allows Users To Input Critical Details, Such As The Investigator’s Name, Contact.
Related Post: