Capa Template
Capa Template - Start using template view template in library. Generate reports from completed checklists. Eliminate paperwork with digital checklists. Management review findings, including trends A very important tool during the capa process is the capa form, especially in highly regulated life science industries. Track progress, manage resources, and improve communication with cascade, the leading strategy execution platform. Web capa report template. Web corrective action and preventive action (capa) plan template. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action analysis. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. Start using template view template in library. Web most medium to large businesses will have various forms of audits such as finance, health and safety or environmental, the audits review processes and usually result in corrective actions needing to be taken. Eliminate. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. Web capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document. Eliminate paperwork with. Web organizations and companies use corrective action plan examples to deal with and prevent any undesirable behaviors and situations at work. Generate reports from completed checklists. Web capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence. Management review findings, including trends Web most medium to large businesses will have various forms of audits such as finance, health and safety or environmental, the audits review processes and usually result in corrective actions needing to be taken. Free to use for up to 10 users. Generate reports from completed checklists. Web create effective capa forms using a simple. Web capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document. Web create effective capa forms using a simple template. With this, you can identify the untoward incidents and undesirable behaviors then. Web create effective capa forms using a simple template. Generate reports from completed checklists. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action analysis. A very important tool during the capa process is the capa form, especially in highly regulated life. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. It includes a problem statement, action steps, a timeline, and a desired outcome. With this, you can identify the untoward incidents and undesirable behaviors then deal with them in the most appropriate way. Web create effective capa forms using a simple template.. Track progress, manage resources, and improve communication with cascade, the leading strategy execution platform. Start using template view template in library. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. Web corrective action and preventive action (capa) plan template. Web capa report template. With this, you can identify the untoward incidents and undesirable behaviors then deal with them in the most appropriate way. Please consult the main clinical research toolkit page for information. Free to use for up to 10 users. Web create effective capa forms using a simple template. Eliminate paperwork with digital checklists. Web corrective action and preventive action (capa) plan template. Web capa report template. Please consult the main clinical research toolkit page for information. Web capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem,. Various events may lead to creation of capa. It includes a problem statement, action steps, a timeline, and a desired outcome. Web organizations and companies use corrective action plan examples to deal with and prevent any undesirable behaviors and situations at work. Eliminate paperwork with digital checklists. In addition, a capa consultant discusses how the essential tool, capa, can provide lasting support to your company. Web capa report template. Please consult the main clinical research toolkit page for information. Management review findings, including trends With this, you can identify the untoward incidents and undesirable behaviors then deal with them in the most appropriate way. Generate reports from completed checklists. A very important tool during the capa process is the capa form, especially in highly regulated life science industries. This resource has been removed. Track progress, manage resources, and improve communication with cascade, the leading strategy execution platform. Web most medium to large businesses will have various forms of audits such as finance, health and safety or environmental, the audits review processes and usually result in corrective actions needing to be taken. Web capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action analysis.
Capa Template Fda

Sample Capa Form
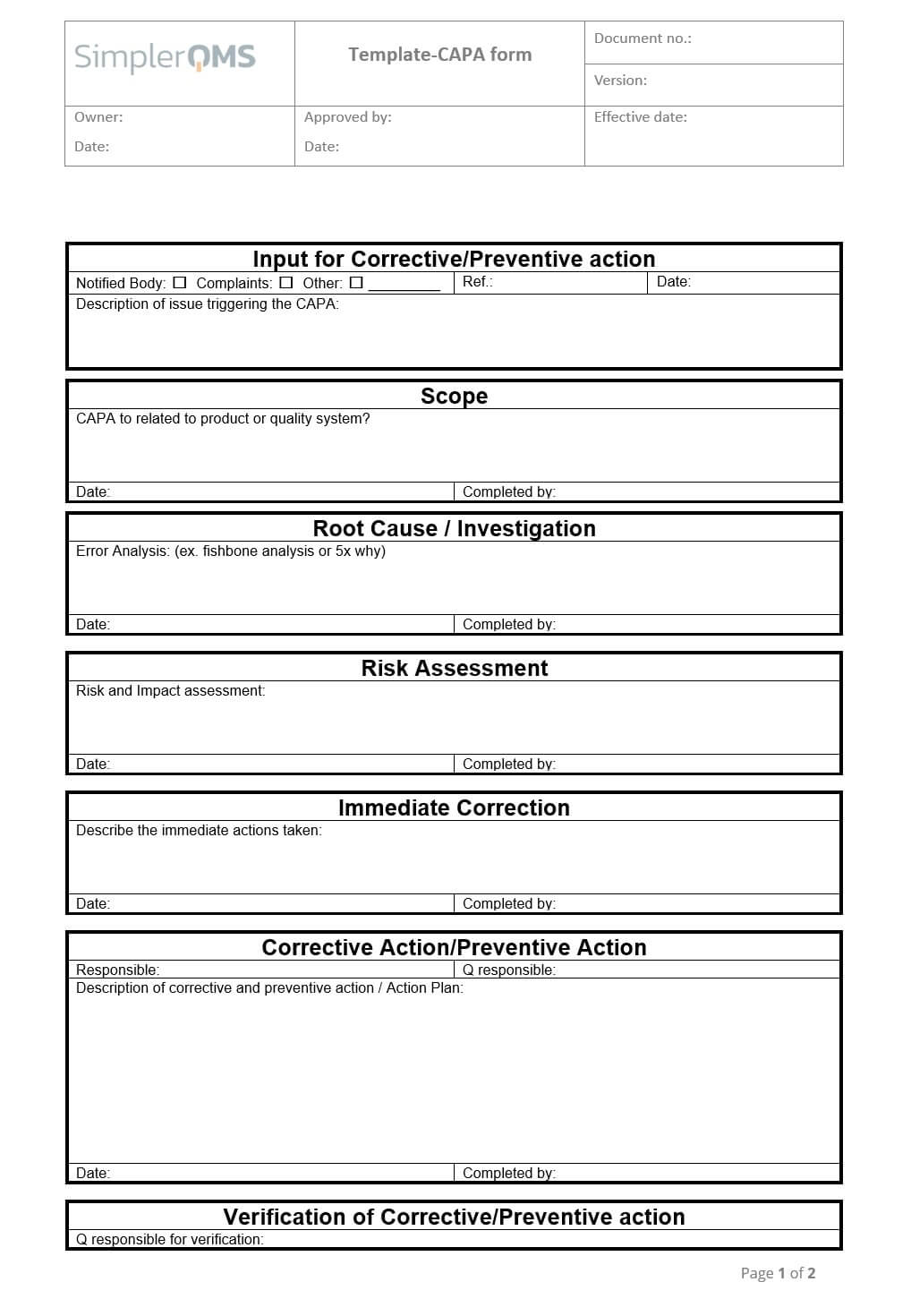
Corrective and Preventive Action (CAPA) Form Template SimplerQMS
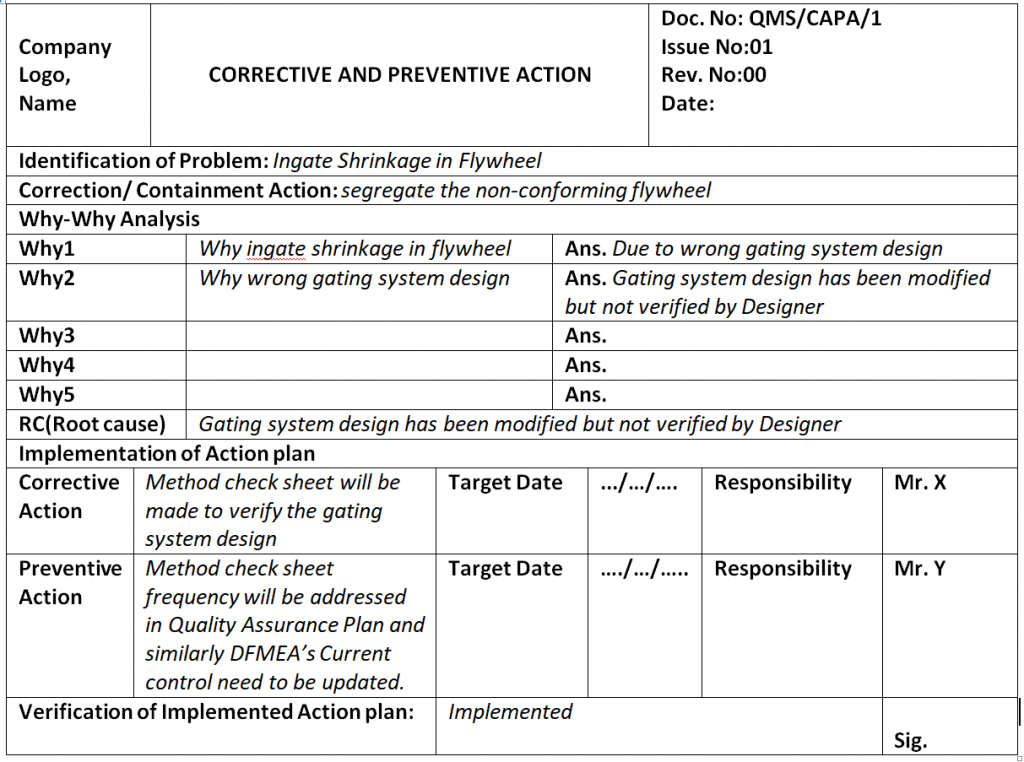
Capa Report Template

Sample Capa Form

CAPA form Corrective action and preventive action
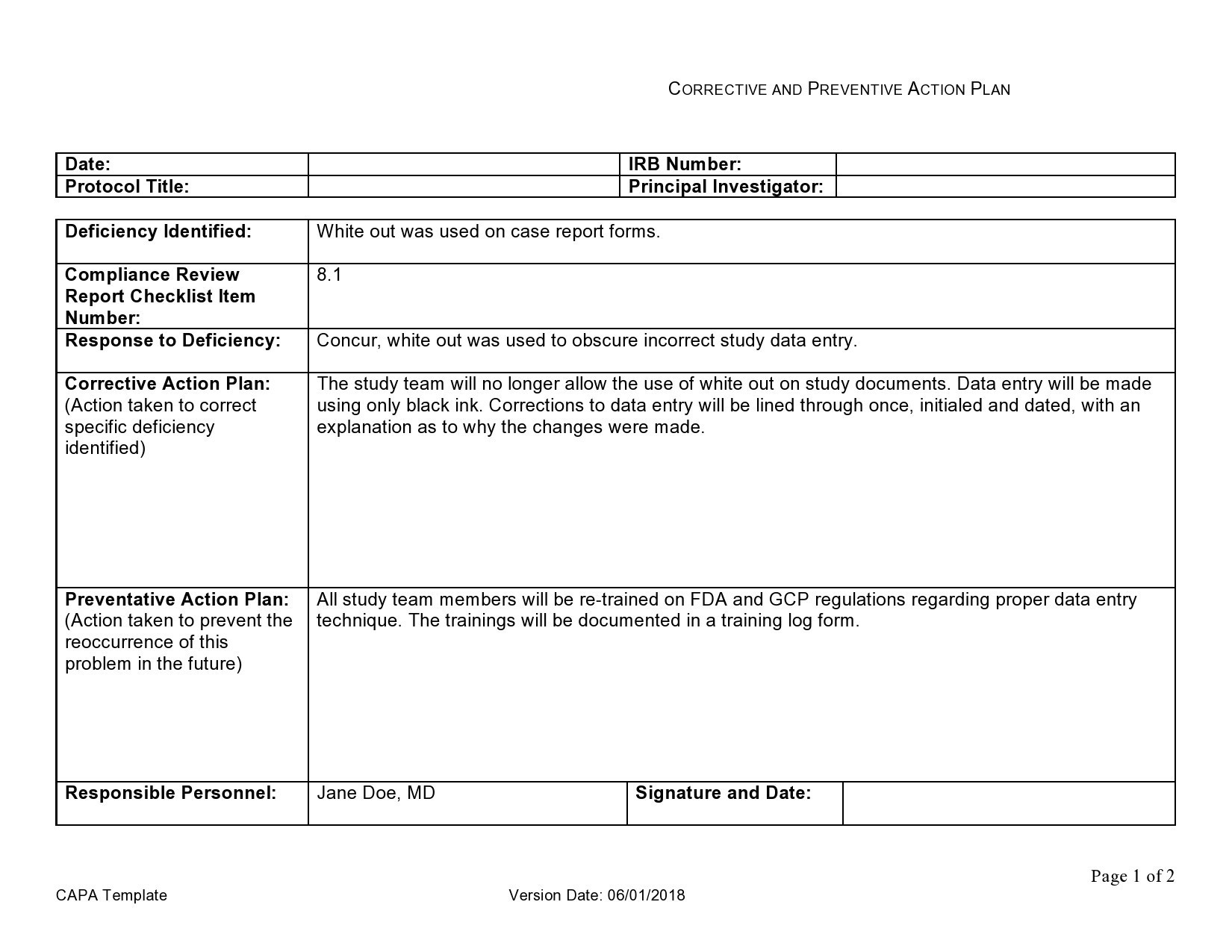
Capa Plan Template
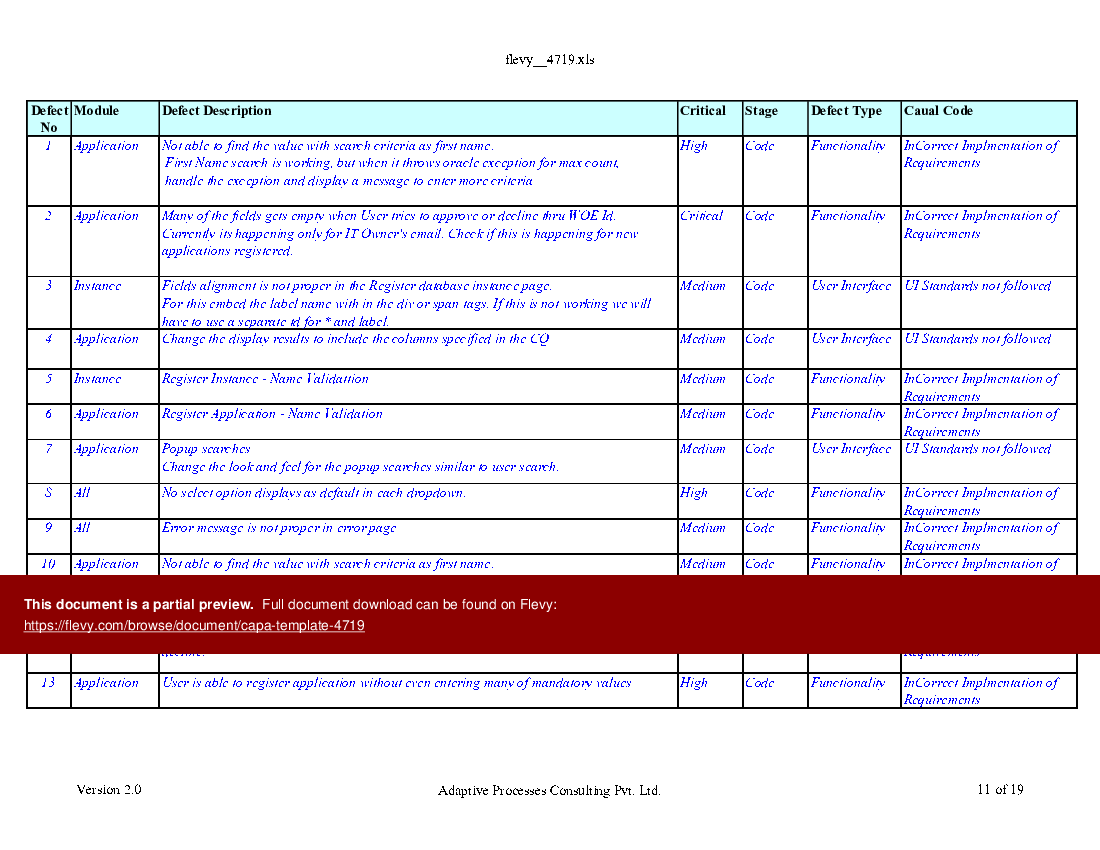
CAPA Template (Excel workbook (XLS)) Flevy
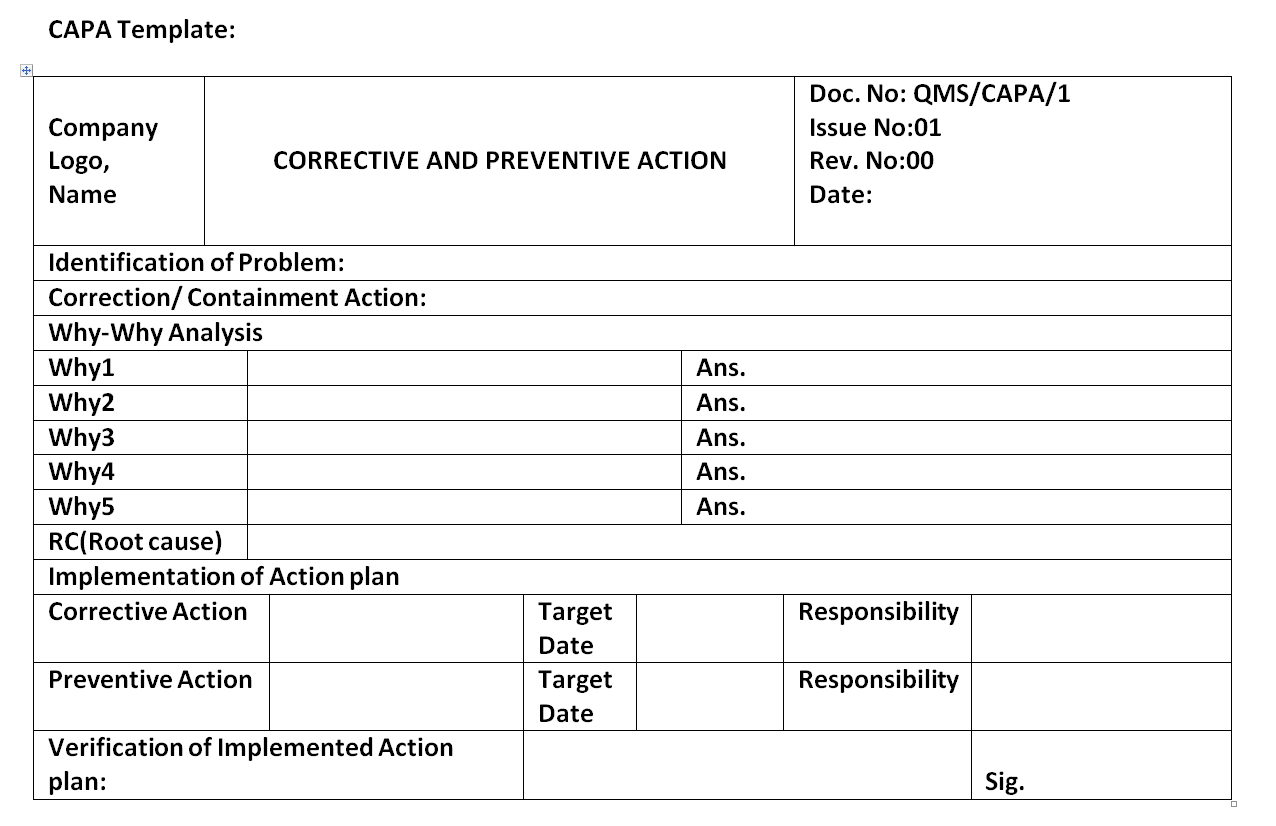
Corrective and Preventive Action Format CAPA with Example
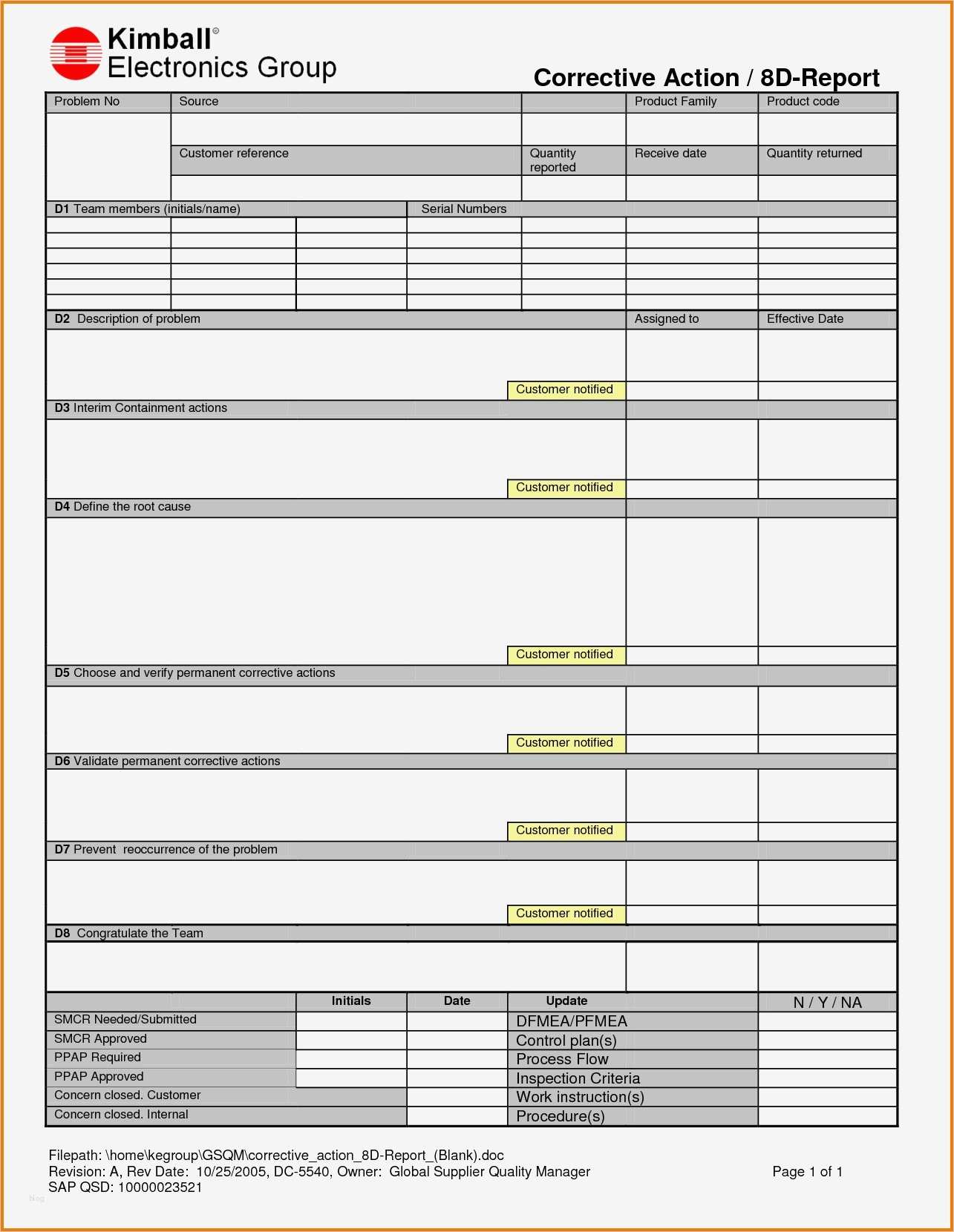
Capa Vorlage Elegant Capa Report Template Awesome Corrective and
Start Using Template View Template In Library.
Web Create Effective Capa Forms Using A Simple Template.
Web Corrective Action And Preventive Action (Capa) Plan Template.
Free To Use For Up To 10 Users.
Related Post: