Breakthrough Therapy Designation
Breakthrough Therapy Designation - Breakthrough therapy is a united states food and drug administration designation that expedites drug development that was created by congress under section 902 of the 9 july 2012 food and drug administration safety and innovation act. Drugs may be listed more than once since breakthrough therapy. New reports will be published. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the basis of 89 pivotal trials. Web the cder breakthrough therapy (bt) approvals reports contain a list of approvals for breakthrough therapy designated drugs. Web “remarkably, the fda — after reviewing our preliminary data — contacted us to urge us to apply for breakthrough therapy designation, rather than waiting for us to. These regulations allowed drugs for serious conditions that filled an unmet medical need to be approved based on a surrogate. Find out the criteria, benefits, and how to request this designation from fda. Web earlier this month, pfizer announced rsvpref received breakthrough therapy designation from the u.s. Web scemblix previously received breakthrough therapy designation for the treatment of newly diagnosed adult patients and is currently being reviewed under the. New reports will be published. Web scemblix previously received breakthrough therapy designation for the treatment of newly diagnosed adult patients and is currently being reviewed under the. These medications are usually for serious medical conditions. Web granted breakthrough device designation and 510(k) clearance by the u.s. Breakthrough therapy is a united states food and drug administration designation that expedites drug. Web granted breakthrough device designation and 510(k) clearance by the u.s. Find out the criteria, benefits, and how to request this designation from fda. Web the cder breakthrough therapy (bt) approvals reports contain a list of approvals for breakthrough therapy designated drugs. The fda granted breakthrough therapy designation based on phase 2. Web earlier this month, pfizer announced rsvpref received. Web breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat serious conditions and when preliminary. Web earlier this month, pfizer announced rsvpref received breakthrough therapy designation from the u.s. Web 23 rows drugs granted breakthrough therapy designation (btd) by the us food and drug administration (fda). Web “remarkably, the fda —. A breakthrough therapy is a drug: Drugs may be listed more than once since breakthrough therapy. Find out the criteria, benefits, and how to request this designation from fda. These medications are usually for serious medical conditions. Web the fda’s breakthrough therapy designation allows for faster medication development. Find out the criteria, benefits, and how to request this designation from fda. Rather, it allows the fda to grant priority review to. The fda granted breakthrough therapy designation based on phase 2. Web the fda’s breakthrough therapy designation allows for faster medication development. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the. A breakthrough therapy is a drug: Web the fda's breakthrough therapy designation is granted to expedite the development and review of drugs in the united states that are intended to treat a. Web 23 rows drugs granted breakthrough therapy designation (btd) by the us food and drug administration (fda). Web breakthrough therapy designation is designed to expedite the development and. Web “remarkably, the fda — after reviewing our preliminary data — contacted us to urge us to apply for breakthrough therapy designation, rather than waiting for us to. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the basis of 89 pivotal trials. These regulations allowed drugs for serious conditions that filled an unmet. A breakthrough therapy is a drug: Intended alone or in combination with. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the basis of 89 pivotal trials. Food & drug administration (fda), saint therapy has led to dramatic improvements in. Rather, it allows the fda to grant priority review to. Find out the criteria, benefits, and how to request this designation from fda. Breakthrough therapy is a united states food and drug administration designation that expedites drug development that was created by congress under section 902 of the 9 july 2012 food and drug administration safety and innovation act. Web learn about breakthrough therapy designation, a process to fast track. Web breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat serious conditions and when preliminary. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the basis of 89 pivotal trials. Intended alone or in combination with. Drugs may be listed more than once since breakthrough. These medications are usually for serious medical conditions. Rather, it allows the fda to grant priority review to. Web learn about breakthrough therapy designation, a process to fast track drugs that may demonstrate substantial improvement over available therapy for a serious condition. New reports will be published. Find out the criteria, benefits, and how to request this designation from fda. Web breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat serious conditions and when preliminary. Web breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat serious conditions and when preliminary. Web from 2012 through 2017, the fda approved 46 therapeutics with breakthrough therapy designation on the basis of 89 pivotal trials. Web “remarkably, the fda — after reviewing our preliminary data — contacted us to urge us to apply for breakthrough therapy designation, rather than waiting for us to. Web 23 rows drugs granted breakthrough therapy designation (btd) by the us food and drug administration (fda). Web the fda's breakthrough therapy designation is granted to expedite the development and review of drugs in the united states that are intended to treat a. Web the cder breakthrough therapy (bt) approvals reports contain a list of approvals for breakthrough therapy designated drugs. Web scemblix previously received breakthrough therapy designation for the treatment of newly diagnosed adult patients and is currently being reviewed under the. Web fast track designation, breakthrough therapy designation, accelerated approval, and priority review designation (referred to in this guidance as the agency’s expedited. Drugs may be listed more than once since breakthrough therapy. The fda granted breakthrough therapy designation based on phase 2.
Breakthrough Therapy Designation 3 Years Later Celgene

BTD request assignment v3 BREAKTHROUGH THERAPY DESIGNATION REQUEST

Infographic Breakthrough Therapy Designation — Three Years Later Celgene

Breakthrough Therapy Designation Oncology Lessons YouTube
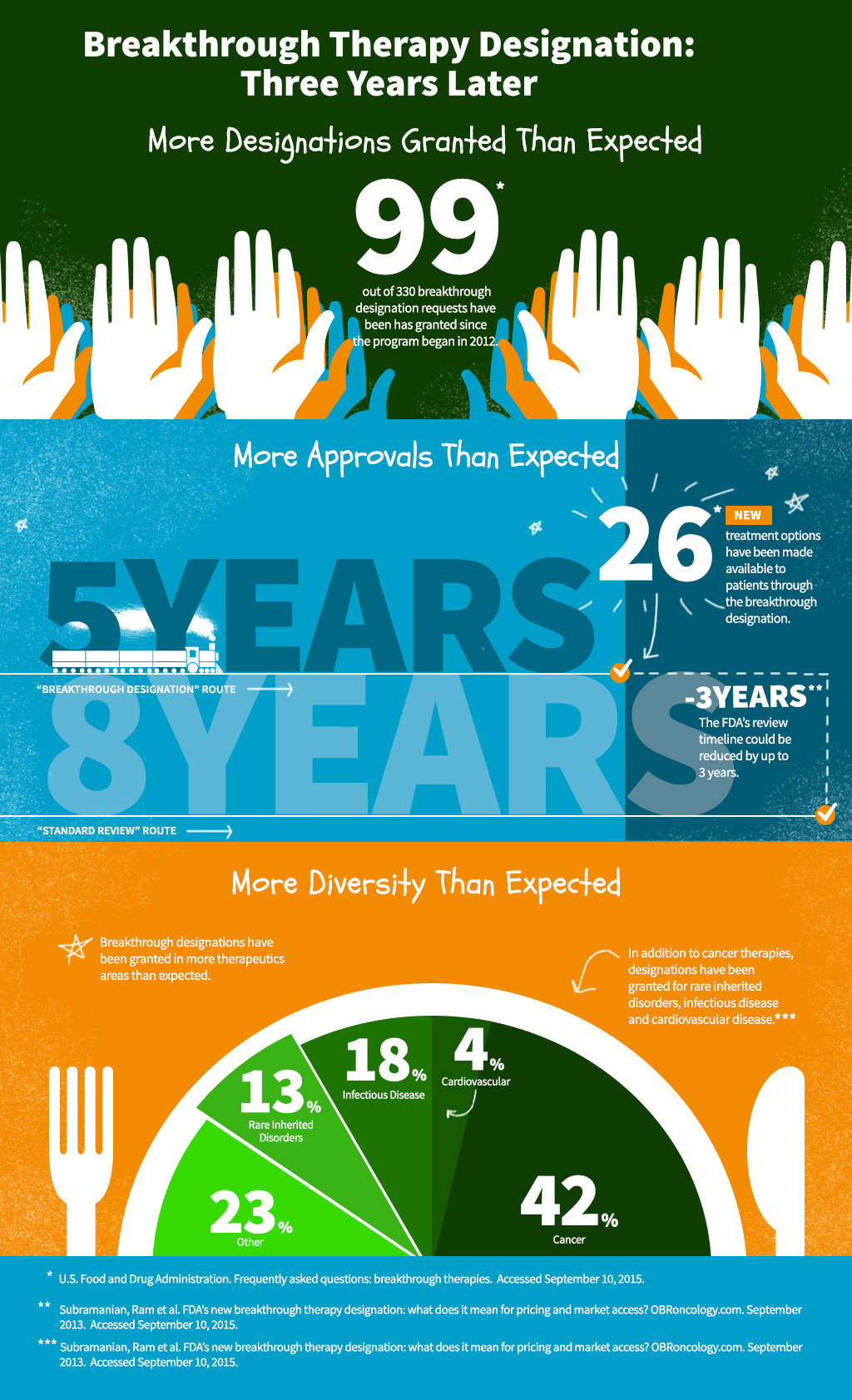
Infographic Breakthrough Therapy Designation — Three Years Later Celgene
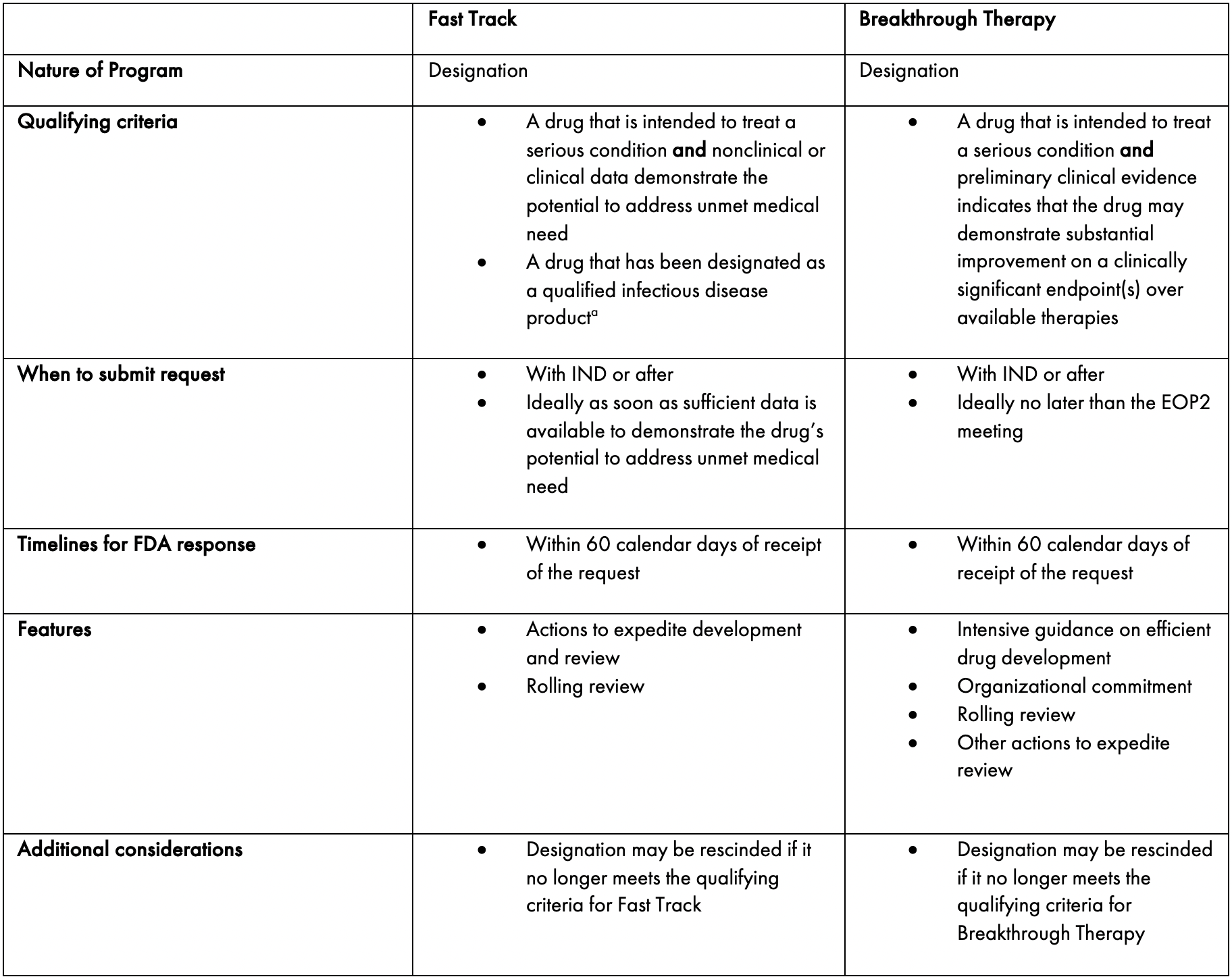
Fast Track Designation and Breakthrough Therapy Designation — Scendea

The Breakthrough Therapy Designation Clearview Clearview

F2G Receives Second US FDA Breakthrough Therapy Designation for
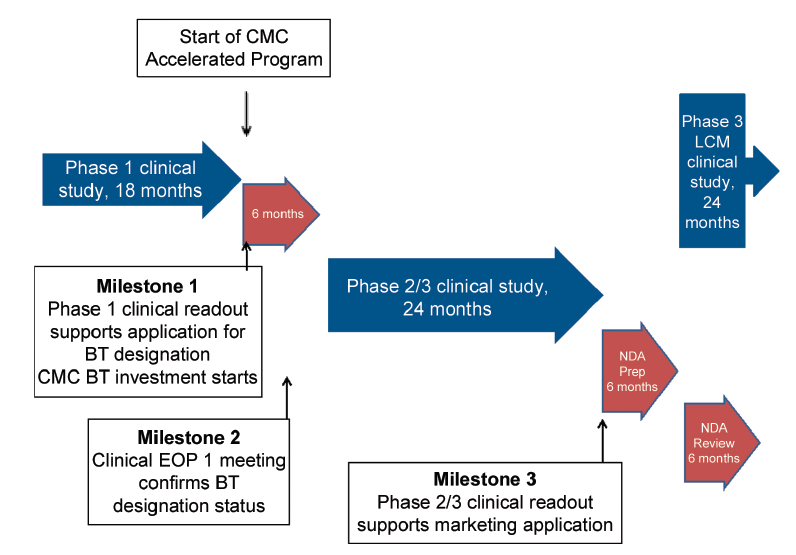
CMC Considerations when a Drug Development Project is Assigned
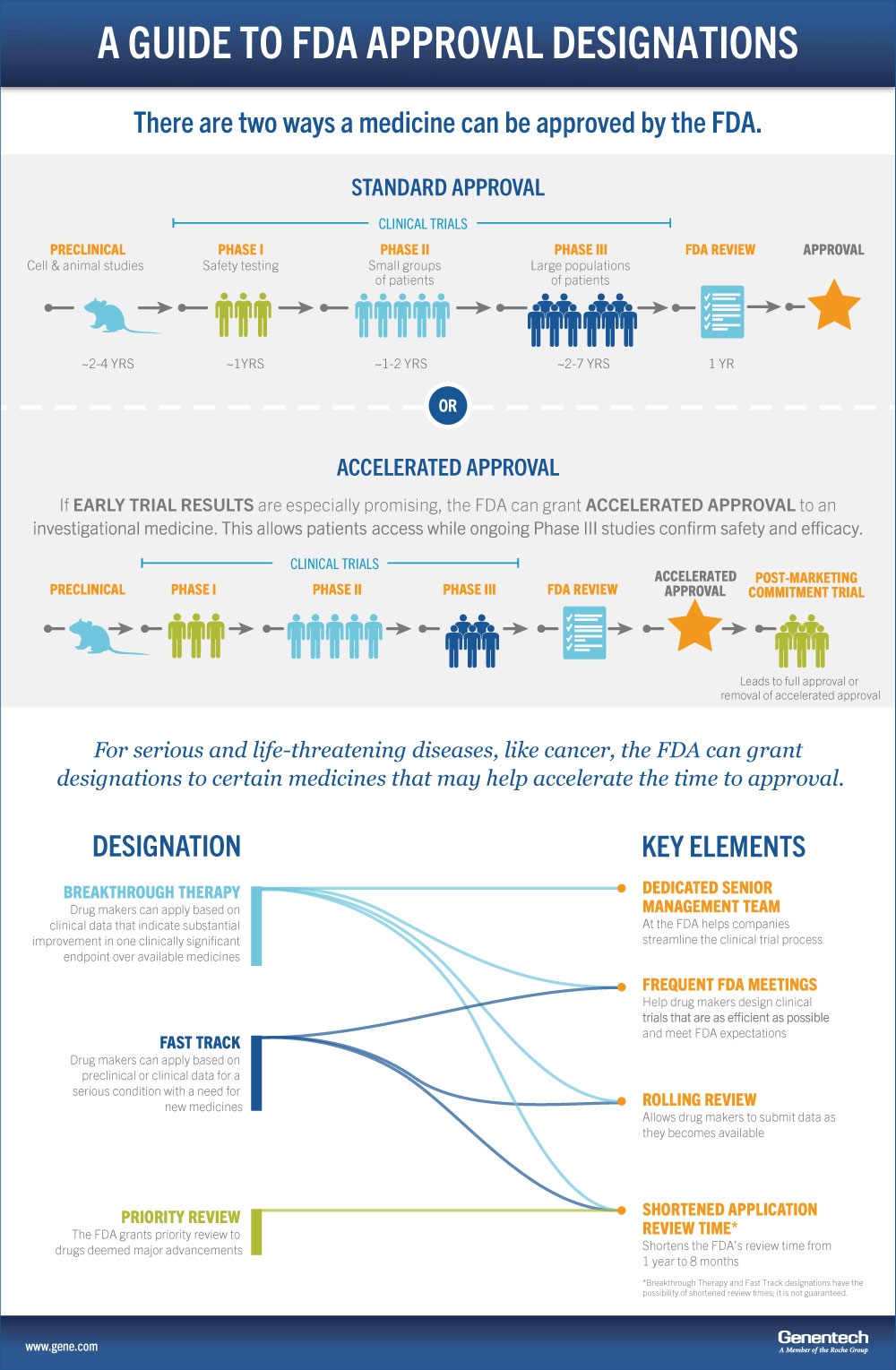
Genentech Breakthrough Designation Explained
Breakthrough Therapy Is A United States Food And Drug Administration Designation That Expedites Drug Development That Was Created By Congress Under Section 902 Of The 9 July 2012 Food And Drug Administration Safety And Innovation Act.
Web Earlier This Month, Pfizer Announced Rsvpref Received Breakthrough Therapy Designation From The U.s.
Web The Fda’s Breakthrough Therapy Designation Allows For Faster Medication Development.
Intended Alone Or In Combination With.
Related Post: