Breakthrough Device Designation
Breakthrough Device Designation - Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web wed, jul 31, 2024, 3:43 pm 2 min read. Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical devices by speeding up development, assessment, and review for. Web the breakthrough designation helps the fda identify new technology to focus on to expedite access to novel devices that will save lives and treat debilitating diseases. This designation helps expedite the development and review of therapies intended to treat. Web we sought to examine five key aspects of early fda experience with the bdp: It takes the fda longer to review these devices because they may raise novel scientific and regulatory issues. Web as of june 30, 2023, cdrh has granted breakthrough device designation to 831 devices and granted marketing authorization to 77 devices with breakthrough device designation. First, the number of devices receiving fda breakthrough designation over time; Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical devices by speeding up development, assessment, and review for. Web we sought to examine five key aspects of early fda experience with the bdp: First, the number of devices receiving fda breakthrough designation over time; This designation helps expedite the development. Web as of june 30, 2023, cdrh has granted breakthrough device designation to 831 devices and granted marketing authorization to 77 devices with breakthrough device designation. Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web the breakthrough designation helps the fda identify new technology to focus on to expedite access to novel devices. Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web the breakthrough designation helps the fda identify new technology to focus on to expedite access to novel devices that will save lives and treat debilitating diseases. It takes the fda longer to review these devices because they may raise novel scientific and regulatory issues.. Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical devices by speeding up development, assessment, and review for. Web the breakthrough designation helps the fda identify new technology to focus on to expedite access to novel devices that will save lives and treat debilitating diseases. First, the number of devices. First, the number of devices receiving fda breakthrough designation over time; Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical devices by speeding up development, assessment, and review for. Web wed, jul 31, 2024, 3:43 pm 2 min read. Web t he food and drug administration designates as “breakthrough” technologies. Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web as of june 30, 2023, cdrh has granted breakthrough device designation to 831 devices and granted marketing authorization to 77 devices with breakthrough device designation. Web wed, jul 31, 2024, 3:43 pm 2 min read. This designation helps expedite the development and review of. This designation helps expedite the development and review of therapies intended to treat. Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web we sought to examine five key aspects of early fda experience with the bdp: First, the number of devices receiving fda breakthrough designation over time; Web the breakthrough devices program is. Web we sought to examine five key aspects of early fda experience with the bdp: First, the number of devices receiving fda breakthrough designation over time; It takes the fda longer to review these devices because they may raise novel scientific and regulatory issues. This designation helps expedite the development and review of therapies intended to treat. Web the breakthrough. Web we sought to examine five key aspects of early fda experience with the bdp: Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. First, the number of devices receiving fda breakthrough designation over time; Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical. Web as of june 30, 2023, cdrh has granted breakthrough device designation to 831 devices and granted marketing authorization to 77 devices with breakthrough device designation. Web the breakthrough designation helps the fda identify new technology to focus on to expedite access to novel devices that will save lives and treat debilitating diseases. This designation helps expedite the development and. It takes the fda longer to review these devices because they may raise novel scientific and regulatory issues. Web as of june 30, 2023, cdrh has granted breakthrough device designation to 831 devices and granted marketing authorization to 77 devices with breakthrough device designation. Web t he food and drug administration designates as “breakthrough” technologies certain drugs and devices. Web wed, jul 31, 2024, 3:43 pm 2 min read. Web we sought to examine five key aspects of early fda experience with the bdp: Web the breakthrough devices program is intended to provide patients and health care providers with timely access to medical devices by speeding up development, assessment, and review for. First, the number of devices receiving fda breakthrough designation over time;
Breakthrough Device Designation Reimbursement

Sana Health Receives FDA "Breakthrough Device Designation" for Sana

Olfactomics Resect received Breakthrough device designation from the

Sooma Sooma Receives FDA Breakthrough Device Designation

SOLVD Health Designed for Change. Designed for Health

Breakthrough Device Designation Timeline Proxima CRO YouTube

Illumina's TruSight NextGeneration Sequencing Assay Receives FDA

Breakthrough Device Designation FDA Timeline Infographic Tools

Breakthrough Device Designation Mastertrial
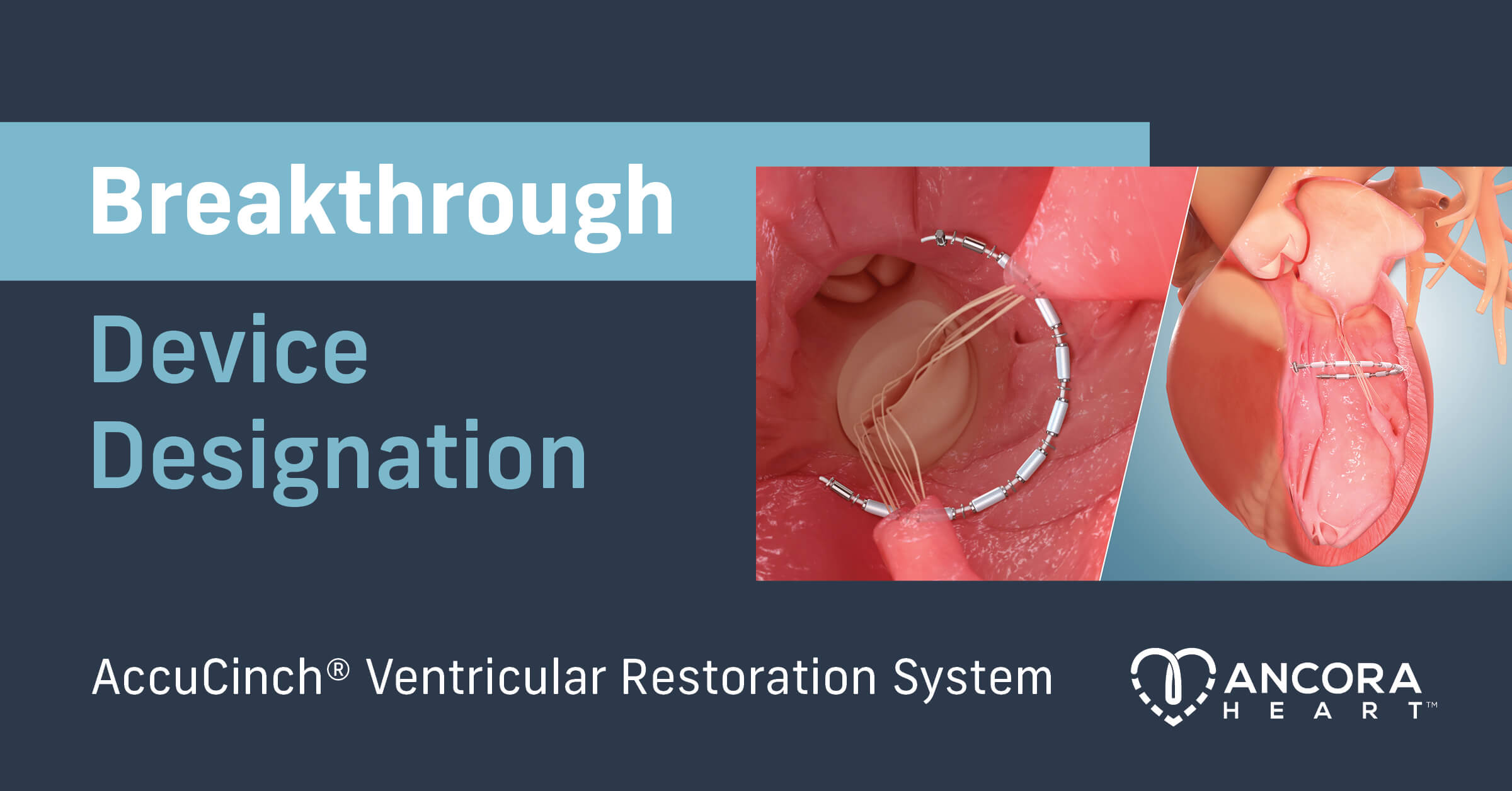
Ancora Heart Receives Breakthrough Device Designation from FDA for the
Web The Breakthrough Designation Helps The Fda Identify New Technology To Focus On To Expedite Access To Novel Devices That Will Save Lives And Treat Debilitating Diseases.
This Designation Helps Expedite The Development And Review Of Therapies Intended To Treat.
Related Post: