Bond Length Chart
Bond Length Chart - Web in chemistry, bond length is the equilibrium distance between the nuclei of two groups or atoms that are bonded to each other. Bonds vary between atoms depending on the molecule that contains them. Watch a video lesson from khan academy, a free online learning platform. Web a table with experimental single bonds for carbon to other elements is given below. For this example, the radius of atom a is 24 picometers and the radius of atom b is 32 picometers. It is clear that as the covalent radius of the elements decreases, the bond length also decreases. Web discover how bond length and bond energy are related to the strength of covalent bonds. C=c > c=n > c=o. To determine the bond order between two covalently bonded atoms, follow these steps: Web this content is for registered users only. C=c > c=n > c=o. It is clear that as the covalent radius of the elements decreases, the bond length also decreases. Bond lengths are given in picometers.by approximation the bond distance between two different atoms is the sum of the individual covalent radii (these are given in the chemical element articles for each element). Web this content is for. Web how to calculate bond length? This is because as we go down the group, the atomic radius increases. Bonds vary between atoms depending on the molecule that contains them. Khan academy offers free online courses on various topics in inorganic chemistry, such as periodic trends, lewis structures, vsepr theory, hybridization, and molecular orbital theory. Bond length is a property. Web the order of bond lengths of carbon and other atoms are as follows: It is clear that as the covalent radius of the elements decreases, the bond length also decreases. Web how to calculate bond length? Bond order is the number of bonding pairs of electrons between two atoms. Web in chemistry, bond length is the equilibrium distance between. Bond lengths are given in picometers.by approximation the bond distance between two different atoms is the sum of the individual covalent radii (these are given in the chemical element articles for each element). C=c > c=n > c=o. In a covalent bond between two atoms, a single bond has a bond order of one, a double bond has a bond. In a covalent bond between two atoms, a single bond has a bond order of one, a double bond has a bond order of two, a triple bond has a bond order of three, and so on. Web how to calculate bond length? First, determine the lengths of the covalent radii. Web below is a table of average bond lengths.. Web discover how bond length and bond energy are related to the strength of covalent bonds. Bond order is the number of bonding pairs of electrons between two atoms. Khan academy offers free online courses on various topics in inorganic chemistry, such as periodic trends, lewis structures, vsepr theory, hybridization, and molecular orbital theory. This is because as we go. In a covalent bond between two atoms, a single bond has a bond order of one, a double bond has a bond order of two, a triple bond has a bond order of three, and so on. Web below is a table of average bond lengths. Bond lengths are given in picometers.by approximation the bond distance between two different atoms. Bond lengths are given in picometers.by approximation the bond distance between two different atoms is the sum of the individual covalent radii (these are given in the chemical element articles for each element). Web how to calculate bond length? First, determine the lengths of the covalent radii. Web a table with experimental single bonds for carbon to other elements is. Web below is a table of average bond lengths. Bond order is the number of bonding pairs of electrons between two atoms. Web in chemistry, bond length is the equilibrium distance between the nuclei of two groups or atoms that are bonded to each other. Bond lengths are given in picometers.by approximation the bond distance between two different atoms is. Web this content is for registered users only. Web discover how bond length and bond energy are related to the strength of covalent bonds. Web how to calculate bond length? Khan academy offers free online courses on various topics in inorganic chemistry, such as periodic trends, lewis structures, vsepr theory, hybridization, and molecular orbital theory. C=c > c=n > c=o. Bond length is a property of a chemical bond between types of atoms. Web this content is for registered users only. This is because as we go down the group, the atomic radius increases. Bond lengths are given in picometers.by approximation the bond distance between two different atoms is the sum of the individual covalent radii (these are given in the chemical element articles for each element). Web below is a table of average bond lengths. Web bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Web in chemistry, bond length is the equilibrium distance between the nuclei of two groups or atoms that are bonded to each other. To determine the bond order between two covalently bonded atoms, follow these steps: Web a table with experimental single bonds for carbon to other elements is given below. Bonds vary between atoms depending on the molecule that contains them. For this example, the radius of atom a is 24 picometers and the radius of atom b is 32 picometers. Web how to calculate bond length? First, determine the lengths of the covalent radii. As a general trend, bond distances decrease across the row in the periodic table and increase down. In a covalent bond between two atoms, a single bond has a bond order of one, a double bond has a bond order of two, a triple bond has a bond order of three, and so on. Bond order is the number of bonding pairs of electrons between two atoms.
Selected bond lengths (Å), bond angles ( • ), and intermolecular
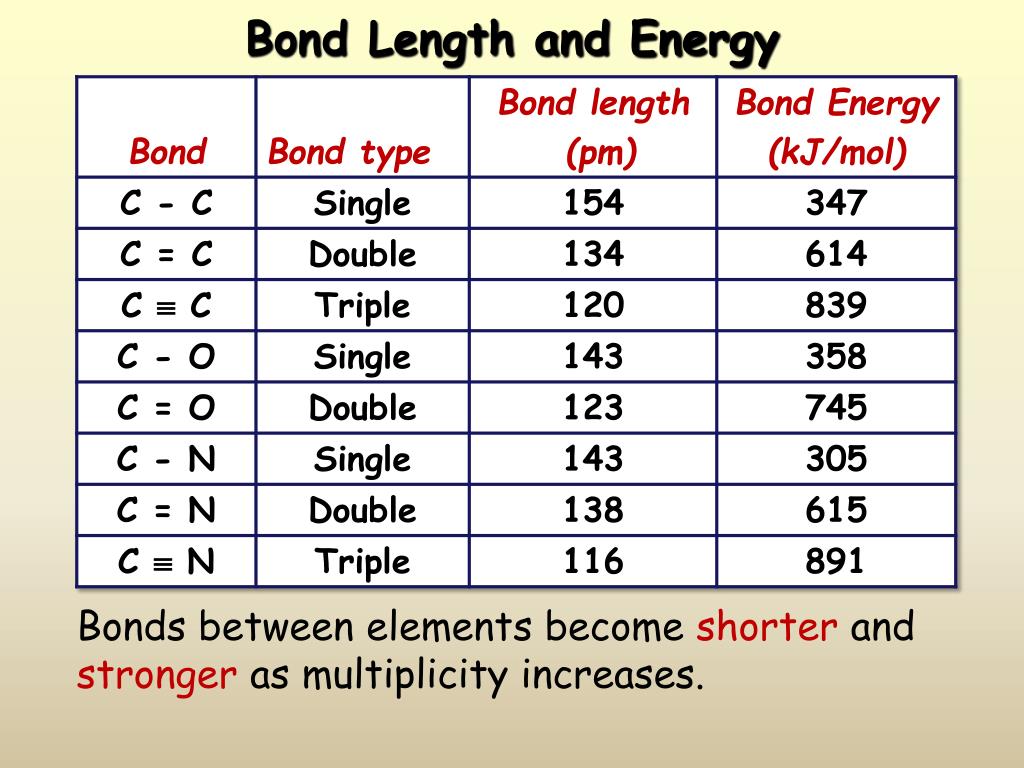
PPT Covalent Bonding PowerPoint Presentation, free download ID1102596

Selected bond lengths (Å) and bond angles (°) for ligand Download Table

PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint

Bond Length and Bond Strength Pathways to Chemistry
Bond Length and Bond Energy

Bond Length and Bond Strength Chemistry Steps
![Bond lengths [Å] and angles [°] for 4c Download Scientific Diagram](https://www.researchgate.net/publication/333256073/figure/download/tbl2/AS:761180935159811@1558491085331/Bond-lengths-A-and-angles-for-4c.png)
Bond lengths [Å] and angles [°] for 4c Download Scientific Diagram

Bond Length and Bond Strength Chemistry Steps
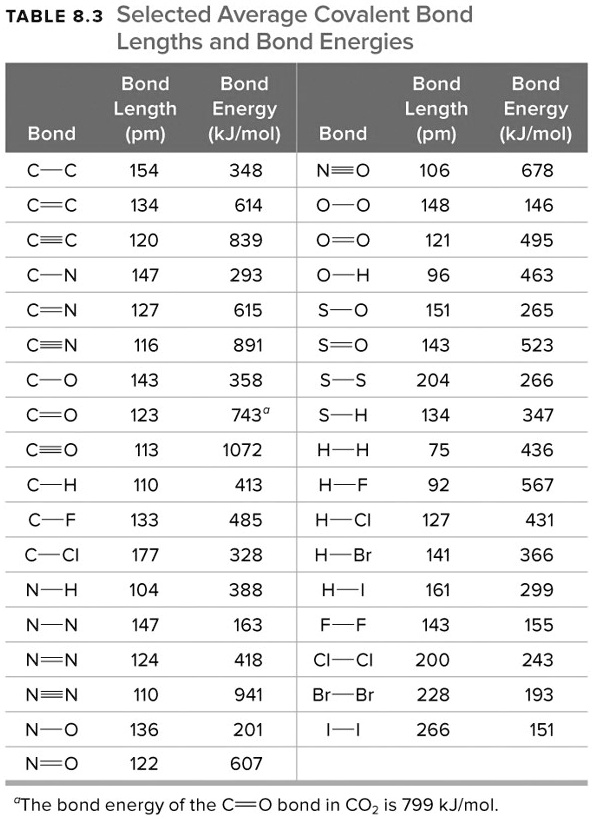
SOLVEDTABLE 8.3 Selected Average Covalent Bond Lengths and Bond
It Is Clear That As The Covalent Radius Of The Elements Decreases, The Bond Length Also Decreases.
Watch A Video Lesson From Khan Academy, A Free Online Learning Platform.
Web Discover How Bond Length And Bond Energy Are Related To The Strength Of Covalent Bonds.
Web Learn About The Different Types Of Chemical Bonds And How They Affect The Structure And Properties Of Molecules.
Related Post:
.PNG)