Batch Manufacturing Record Template
Batch Manufacturing Record Template - Web this template allows batch managers to record important details such as batch id, product name, batch size, production date, batch manager, quality check results, start time, end time, and any additional notes. Web 21 cfr 111.255 defines the requirements for your batch production records: You will learn how to create and issue a bpr, how to fill out the manufacturing instructions and how to use inventory during manufacturing. You will need both roles in creating and issuing bprs. Web the document provides a template for a batch manufacturing record (bmr) that must document every step of the manufacturing process from obtaining raw materials to final packaging. Documenting all materials and equipment used. Overview of batch production records 1. These records typically include detailed instructions on how to manufacture a product, quality control procedures, test. This free batch manufacturing tool will allow you to record all the important details about your production batch for true traceability. The bpr captures all details of the production for a specific batch, ensuring that the process adheres to the defined standards. Follow the master manufacturing record guidelines. Web download our free batch manufacturing spreadsheet template for excel, numbers and google sheets. This free batch manufacturing tool will allow you to record all the important details about your production batch for true traceability. You will learn how to create and issue a bpr, how to fill out the manufacturing instructions and how. Web a batch manufacturing record (bmr) is a document that provides a detailed record of the manufacturing process for a specific batch of a drug product. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. These records typically include detailed instructions on how to manufacture a product, quality control procedures, test. 1) batch details like. Web batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. Web a batch manufacturing record (bmr) is a comprehensive document that outlines the entire production process for a specific batch of a product. It contains all the information related to the production of the batch, including the equipment used, raw materials. Develop a detailed batch record template outlining all necessary information. It details the ingredients, manufacturing process, yield reconciliation, and quality checks for a specific batch. Web a batch manufacturing record or bmr is the controlled document which includes all the information & written instructions that must be followed step by step during the manufacturing of a batch. Web a batch. Web a batch record is a comprehensive set of documents that outlines all aspects of the manufacturing process for a particular batch. Web batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. The bpr captures all details of the production for a specific batch, ensuring that the process adheres to the. Web this template allows batch managers to record important details such as batch id, product name, batch size, production date, batch manager, quality check results, start time, end time, and any additional notes. 1) batch details like number, composition, storage conditions and dates, 2) a list of raw materials and quantities used, 3) safety guidelines. It includes information on the. Prepare a bpr for every batch of dietary supplements manufactured. Develop a detailed batch record template outlining all necessary information. Web the document provides a template for a batch manufacturing record (bmr) that must document every step of the manufacturing process from obtaining raw materials to final packaging. It contains all the information related to the production of the batch,. Web send batch manufacturing record template via email, link, or fax. Web a batch manufacturing record (bmr) is a document that provides a detailed record of the manufacturing process for a specific batch of a drug product. Batch manufacturing records typically include the inputs needed for producing the batch, detailed instructions on how to make the product, quality control procedures. Prepare a bpr for every batch of dietary supplements manufactured. It serves as a detailed guideline for manufacturers to follow in order to ensure consistency and quality control throughout the manufacturing process. The bpr captures all details of the production for a specific batch, ensuring that the process adheres to the defined standards. You will learn how to create and. Web standard operating procedure (sop) for preparation, review, approval, issuance, maintenance, and archival of controlled master batch record (mbr) throughout the product lifecycle to meet and maintain sustainable compliance to current good manufacturing practices (cgmps). Web a batch manufacturing record or bmr is the controlled document which includes all the information & written instructions that must be followed step by. Documenting all materials and equipment used. It includes details about ingredients and supplies used, equipment settings,. Web use this batch record template to document all your manufactured product batches, their ingredients, and the sops needed to create them. Web a batch manufacturing record is the document used for tracking batch production throughout the manufacturing lifecycle. You will learn how to create and issue a bpr, how to fill out the manufacturing instructions and how to use inventory during manufacturing. Web 21 cfr 111.255 defines the requirements for your batch production records: Web this document is a batch manufacturing record for an ayurvedic product. It includes information on the materials and equipment used, the production steps taken, and the testing and analysis performed throughout the production process. Web a batch manufacturing record (bmr) is a document that provides a detailed record of the manufacturing process for a specific batch of a drug product. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. Develop a detailed batch record template outlining all necessary information. Learn how to create a bmr and download a free template. 1) batch details like number, composition, storage conditions and dates, 2) a list of raw materials and quantities used, 3) safety guidelines. In this section, you will learn about batch production records. Web the document provides a template for a batch manufacturing record (bmr) that must document every step of the manufacturing process from obtaining raw materials to final packaging. These records typically include detailed instructions on how to manufacture a product, quality control procedures, test.
Pharmaceutical Batch Manufacturing Record Template
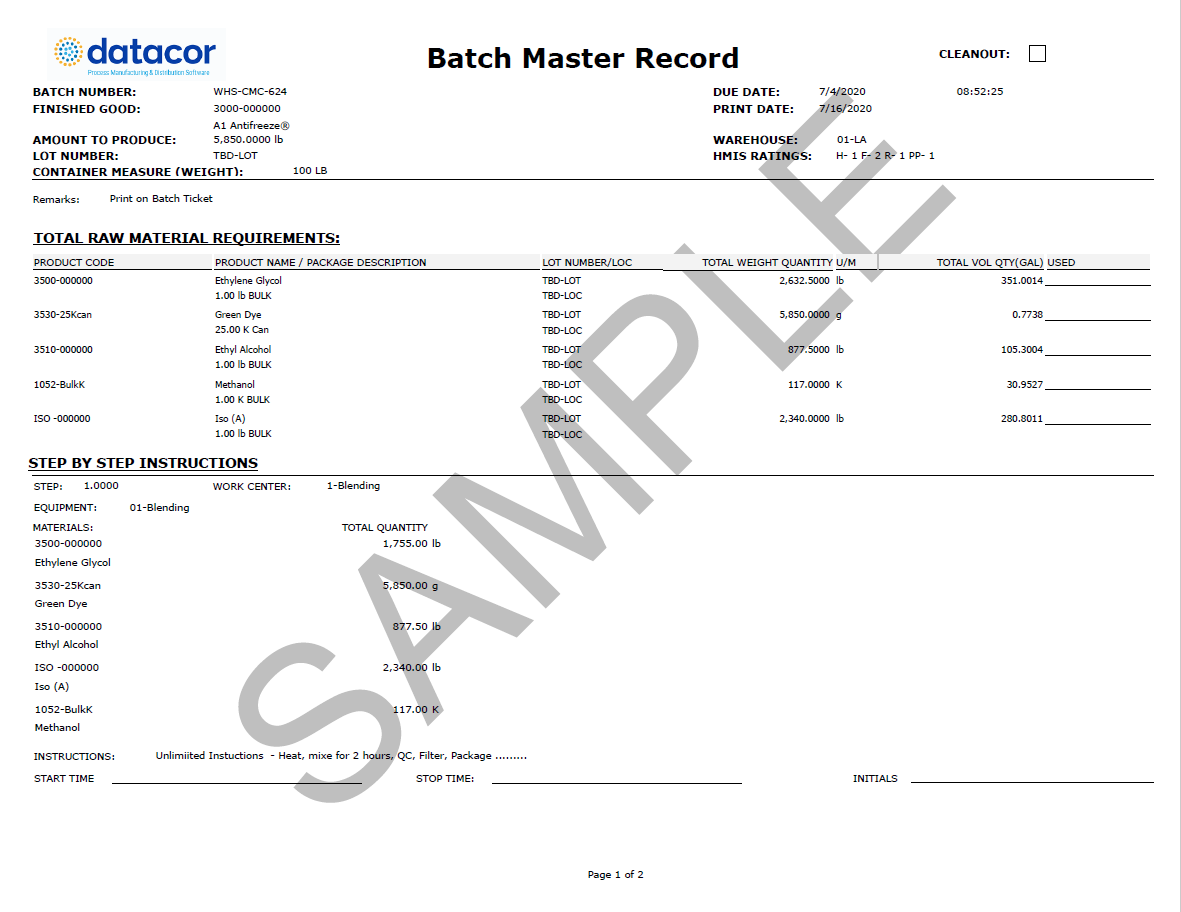
2024 Batch Manufacturing Record Definition, Template & Examples

Electronic Batch Record (EBR) Batch Manufacturing Record (MBR)
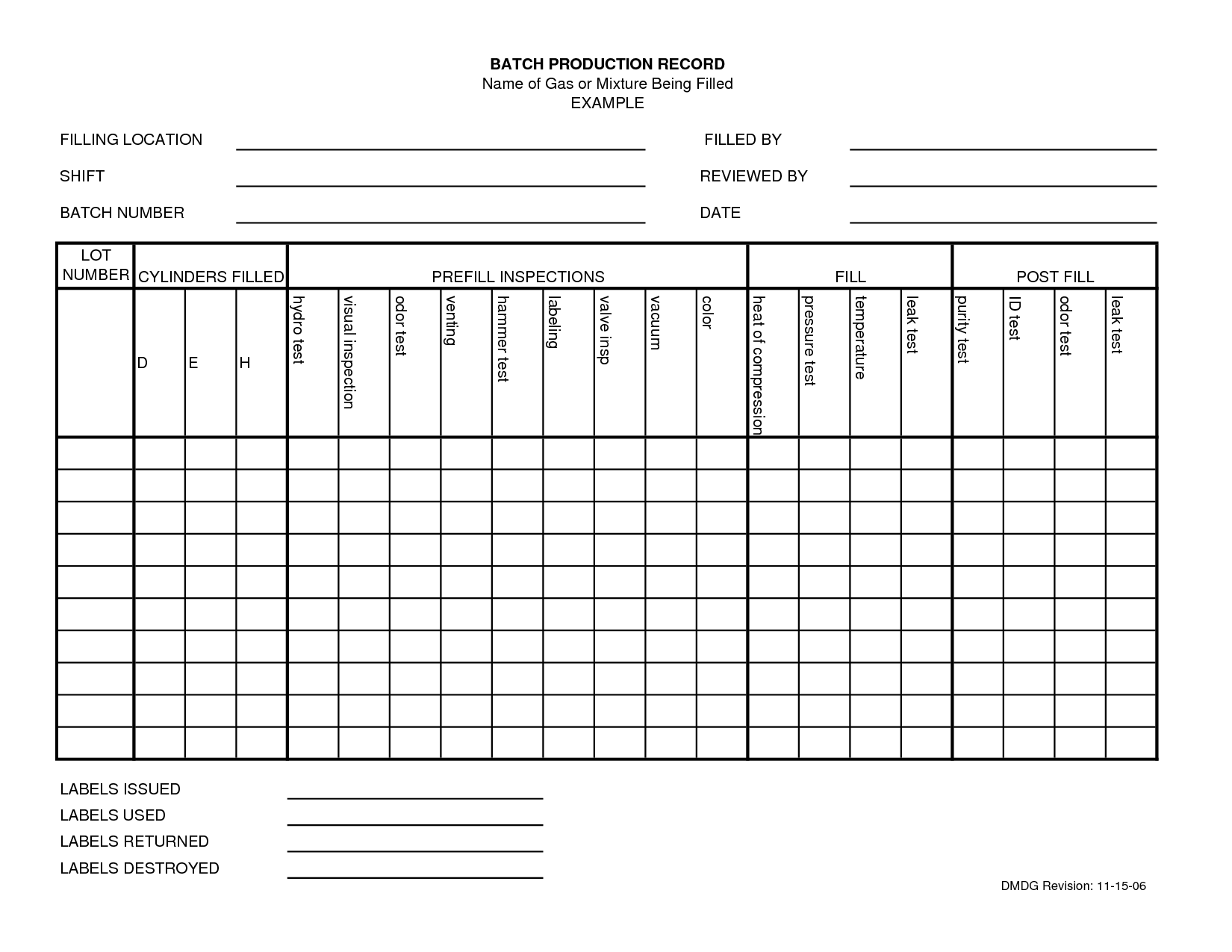
Food Manufacturing Batch Record Template

Batch Record Template Master of Documents
Batch Production Record Templates

Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF

Good Manufacturing Practices Formula & Batch Records
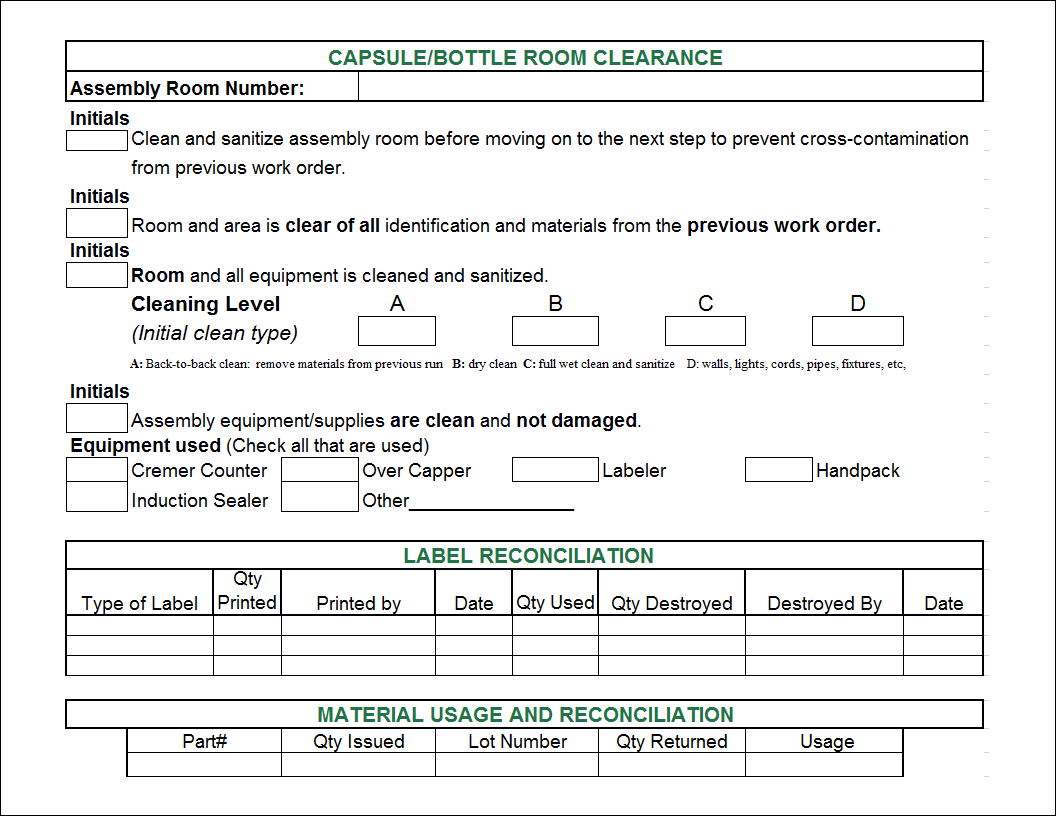
Batch Record Template
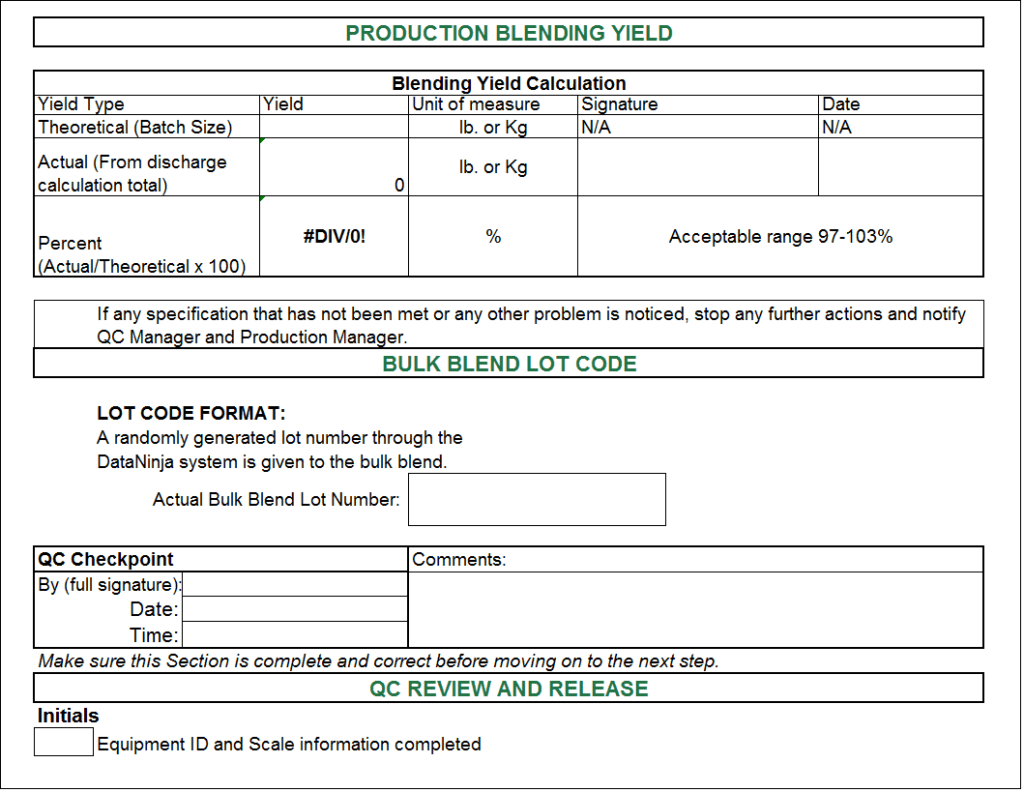
Batch Record Template Excel
Web A Batch Manufacturing Record (Bmr) Is A Comprehensive Document That Outlines The Entire Production Process For A Specific Batch Of A Product.
Web A Batch Production Record (Bpr) Is A Document That Provides A Detailed Record Of The Production Process For A Specific Batch Of A Pharmaceutical Product.
It Contains Actual Data Of The Batch Manufacturing And Whole Manufacturing Process Step By Step.
The Batch Record Manufacturing Process Involves:
Related Post: