Atomic Structure Chart
Atomic Structure Chart - The history of atomic chemistry. Web by convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Web atomic structure or structure of atom consists of a nucleus having protons and neutrons, electrons revolve around it. It is an icon of chemistry and is widely used in physics and other sciences. These are the parts of the atom. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. Web atomic structure comprises of a nucleus in which protons, neutrons, electrons are present. Web atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Web in this unit, we'll cover some of chemistry's most fundamental topics, including atoms, isotopes, ions, and the periodic table. Learn the structure of atoms from the atomic models by dalton thomson rutherford and bohr. Web figure 2.2.1 2.2. Browse videos, articles, and exercises by topic. Web the periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. It is an icon of chemistry and is widely used in physics and other sciences.. The periodic table, electron shells, and orbitals. The mole and avogadro's number. The nucleus, the center of atom contain proton and neutron, and the outer portion of the atom holds electrons in its orbit around the nucleus [1]. Web we understand now that all atoms can be broken down into subatomic particles: Web atomic structure comprises of a nucleus in. The quantum mechanical model of the atom. Web figure 2.2.1 2.2. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The history of atomic chemistry. We’ll also explore different ways of representing atoms, such as through bohr models. Web the structure of the periodic table of elements can be explained in terms of the total energy, orbital angular momentum, and spin of electrons in an atom. Web an atom is composed of two regions: It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger. Web the periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number, oganesson. They are located in nucleus, and have mass. Web we understand now that all atoms can be broken down into subatomic particles: Web atoms contain up. Web this unit explores the atomic theory of matter, the foundational premise of chemistry. Web the development of modern atomic theory revealed much about the inner structure of atoms. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Web the modern atomic theory establishes the concepts of atoms and how they. Web the modern atomic theory establishes the concepts of atoms and how they compose matter. Protons are positively charged particles of atoms. The development of modern atomic theory revealed much about the inner structure of atoms. Web by convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. These are the parts of. The quantum mechanical model of the atom. Atoms are composed of three main subatomic particles: Browse videos, articles, and exercises by topic. The neutron, proton, and electron. Web atoms contain up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). Web the development of modern atomic theory revealed much about the inner structure of atoms. The periodic table, electron shells, and orbitals. The structure of the atom. We’ll also explore different ways of representing atoms, such as through bohr models and lewis diagrams. Web we understand now that all atoms can be broken down into subatomic particles: Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. The mole and avogadro's number. It was learned that an atom contains a very small nucleus. Atoms are composed of three main subatomic particles: Learn about moles and molar mass, mass spectrometry, electron configurations, periodic trends, and more. Web the development of modern atomic theory revealed much about the inner structure of atoms. Web figure 2.2.1 2.2. All of these contribute to the mass and properties of the atom. Web the structure of the periodic table of elements can be explained in terms of the total energy, orbital angular momentum, and spin of electrons in an atom. Web the periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (periods) and columns (groups). Web atomic structure comprises of a nucleus in which protons, neutrons, electrons are present. This unit is part of the chemistry library. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Web by convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Table \(\pageindex{1}\) lists some of their important characteristics and the symbols used to represent each particle. Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Web atoms contain up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). We’ll also explore different ways of representing atoms, such as through bohr models and lewis diagrams. The quantum mechanical model of the atom.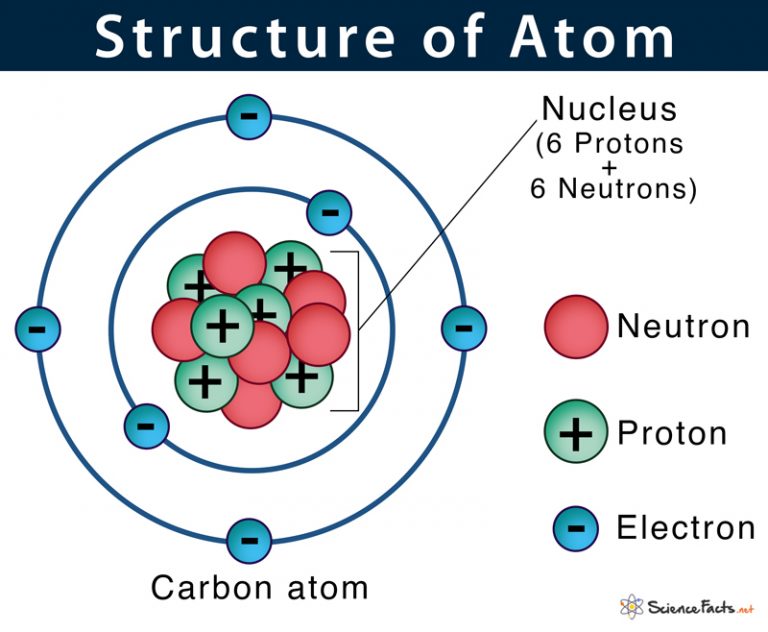
Atom Definition, Structure & Parts with Labeled Diagram

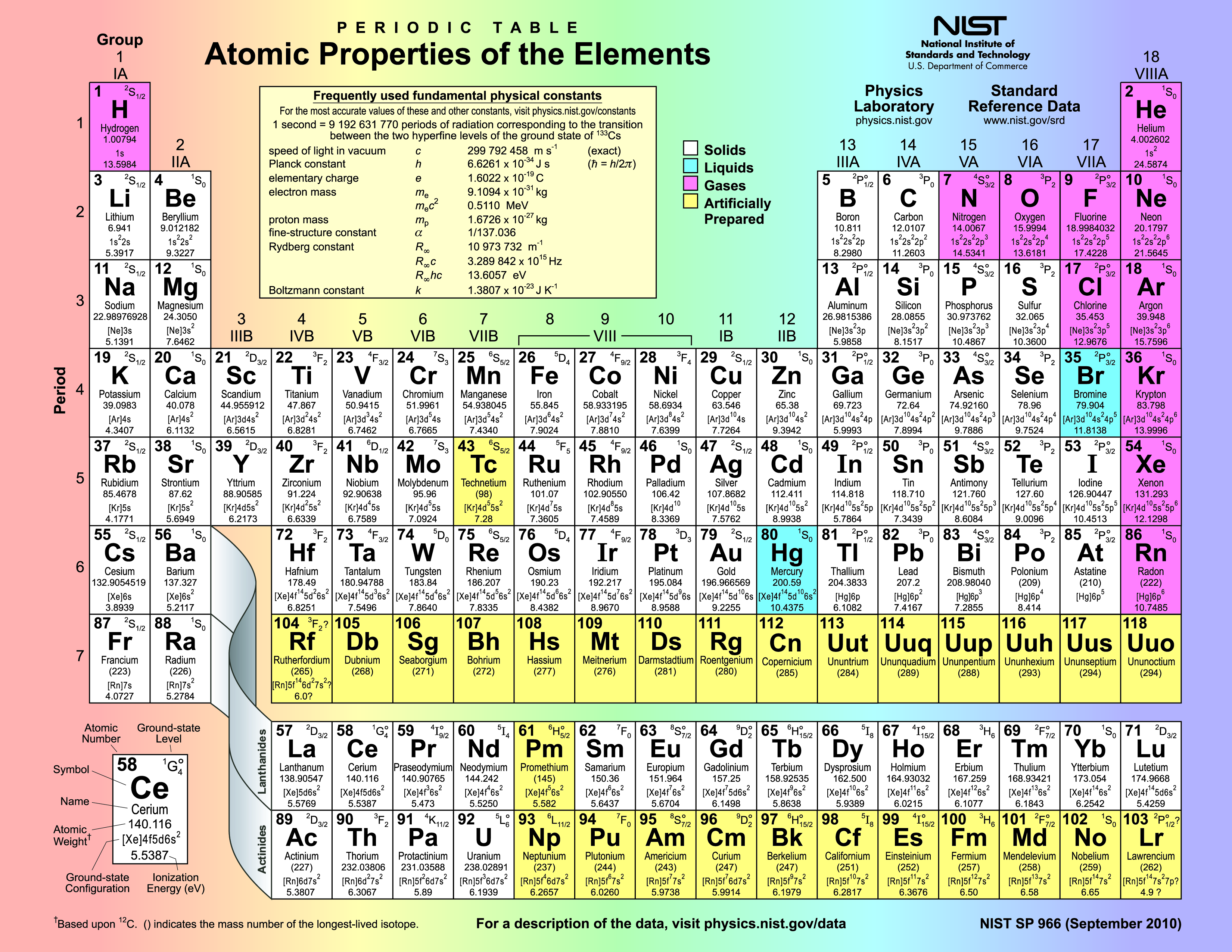
Chapter 1 Atomic Structure And The Periodic Table Periodic Table
Atomic structure infographic as diagram for chemistry study 9016596
Atoms and Atomic Structure HubPages
FilePeriodic Table Atomic Properties of the Elements.png
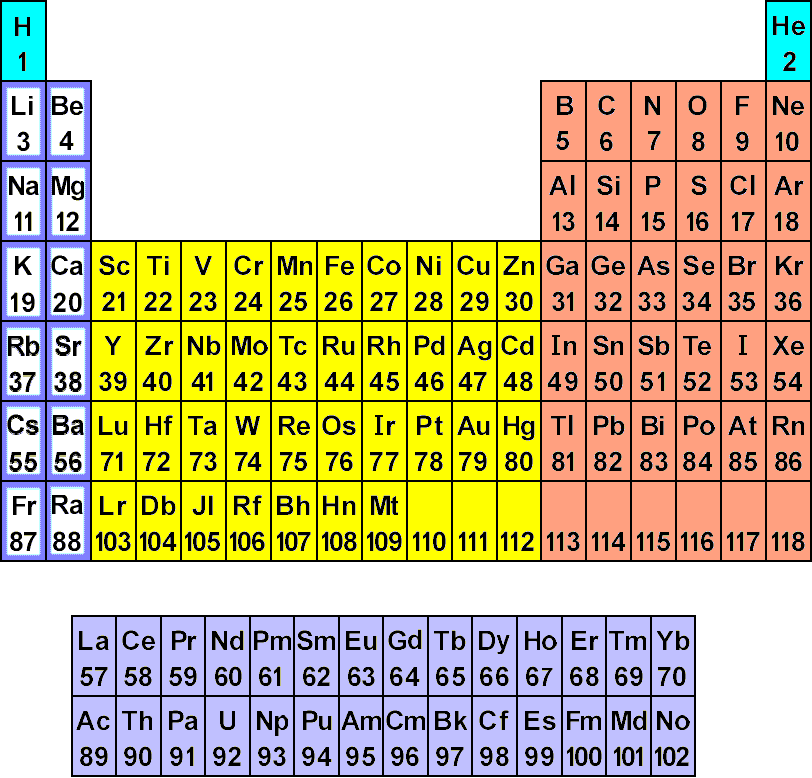
Atomic Structure

Periodic Table Of Elements Structure
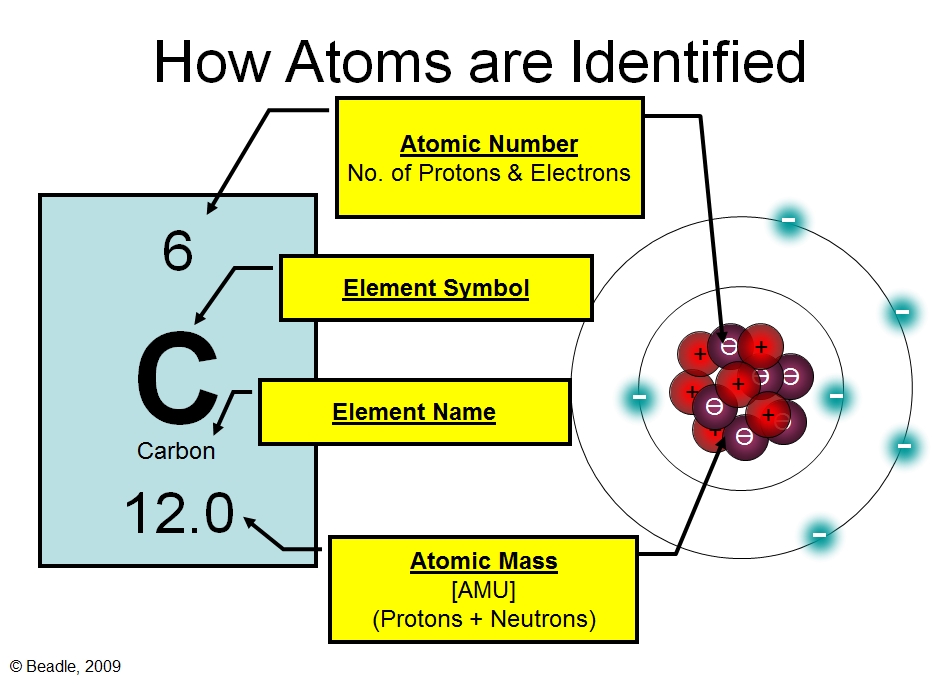
The Atomicstructure Chart Of The Element

Atomic Structures & the Periodic Table VISTA HEIGHTS 8TH GRADE SCIENCE

Atomic Structure, Atomic Mass, Atomic Number ⋆
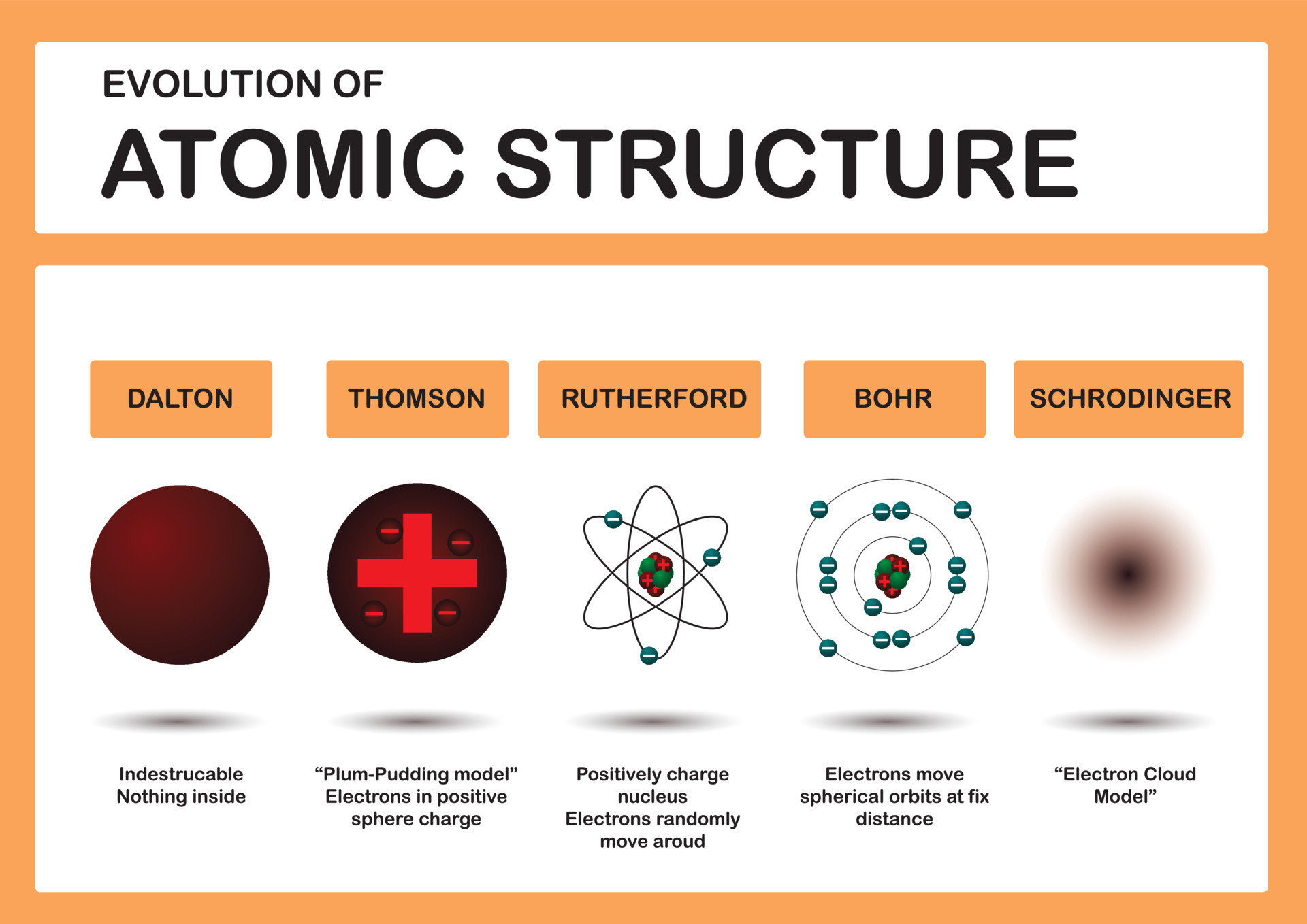
Learn The Structure Of Atoms From The Atomic Models By Dalton Thomson Rutherford And Bohr.
Web We Understand Now That All Atoms Can Be Broken Down Into Subatomic Particles:
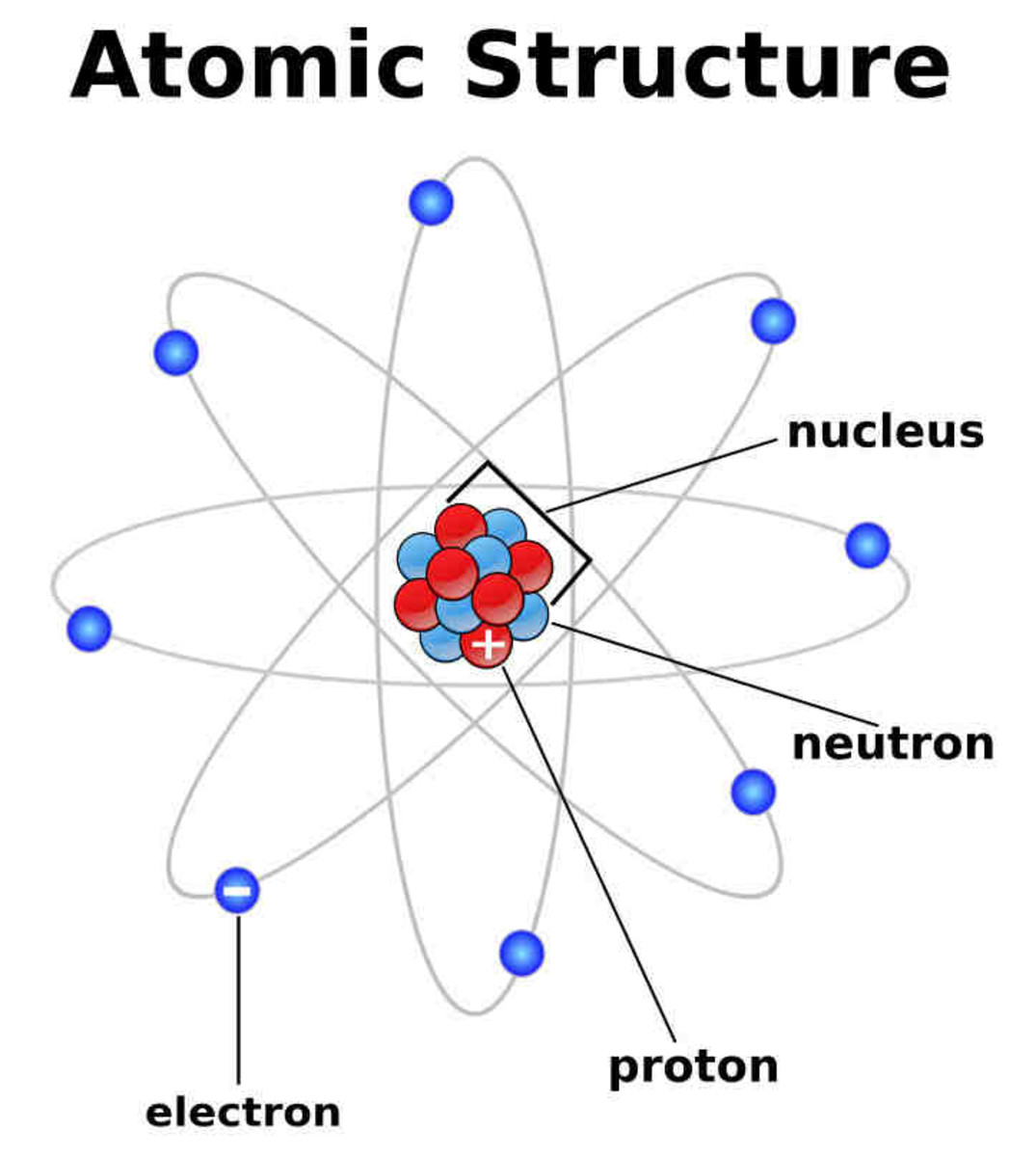
The Nucleus, The Center Of Atom Contain Proton And Neutron, And The Outer Portion Of The Atom Holds Electrons In Its Orbit Around The Nucleus [1].
Understand The Structure Of Atom With Dalton’s, Thomson, Rutherford, And Bohr’s Atomic Model.
Related Post: