An Ice Chart Is Needed To Calculate K C If
An Ice Chart Is Needed To Calculate K C If - For 2024 that limit is $22,320. H 2 o( g ) + co( g ) ⇆ h 2 ( g ) + co 2 ( g ) calculate the number of h 2 moles. Then, we recall the definition of the concentration. Web an useful tool in solving equilibrium problems is an ice chart. 2 hcl in an experiment carried out at 20 o c, the initial Web an ice table is a tool used to calculate the changing concentrations of reactants and products in (dynamic) equilibrium reactions. Web calculate k c for the following reaction at 300 k where the equilibrium concentrations are [so 3] = 0.400 m, [so 2] = 0.200 m, and [o 2] = 0.600 m. Web use an ice table to determine \(k_c\) for the following balanced general reaction: Web an ice chart is a means of organizing data when solving a problem for an equilibrium reaction. This video contains the solution to the following question. Web an useful tool in solving equilibrium problems is an ice chart. \[ \ce{ 2x(g) <=> 3y(g) + 4z(g)} \nonumber\] where the capital letters represent the products and reactants. Web calculate k c for the following reaction at 300 k where the equilibrium concentrations are [so 3] = 0.400 m, [so 2] = 0.200 m, and [o 2] = 0.600. If only equilibrium concentrations are. First, we need to write the balanced chemical equation for the reaction. Then, we recall the definition of the concentration. H 2 o( g ) + co( g ) ⇆ h 2 ( g ) + co 2 ( g ) calculate the number of h 2 moles. Web if you are under full retirement. This method first lists the concentrations of. Acetylene undergoes dimerization to produce vinyl acetylene (butenyne). Web when finding equilibrium concentrations using an equilibrium constant and initial concentrations, it's often helpful to write out the reaction and construct an ice. Web use an ice table to determine \(k_c\) for the following balanced general reaction: To find equilibrium concentrations with ice tables,. If only equilibrium concentrations are. For starters, we (of course) assume ideal gases (these are both gases at room temperature). The key for understanding problem solving is: This method first lists the concentrations of. Web use an ice table to determine \(k_c\) for the following balanced general reaction: For 2024 that limit is $22,320. Then, we recall the definition of the concentration. An ice chart is needed to calculate $k_c$ if: Next, we need to set up an ice (initial, change, equilibrium) chart for the. The key for understanding problem solving is: Web an ice chart is a means of organizing data when solving a problem for an equilibrium reaction. Web the equilibrium constant for the following reaction at 600 o c is determined to be kc = 0.495: An ice chart is needed to calculate $k_c$ if: Web the calculator uses the initial concentration (i), the change in concentration (c), and. For 2024 that limit is $22,320. If only equilibrium concentrations are. Web determining equilibrium concentrations with ice tables. Make an “ice” table, and enter the known initial concentrations. Web an ice chart is a means of organizing data when solving a problem for an equilibrium reaction. Web the calculator uses the initial concentration (i), the change in concentration (c), and the equilibrium concentration (e) to evaluate the state of a chemical equation at equilibrium. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Web it explains how to calculate the equilibrium constant k value given the equilibrium concentrations and equilibrium. Web kc = 0.36. If only equilibrium concentrations are. H 2 o( g ) + co( g ) ⇆ h 2 ( g ) + co 2 ( g ) calculate the number of h 2 moles. To find equilibrium concentrations with ice tables, we need three things: Then, we recall the definition of the concentration. Then, we recall the definition of the concentration. Web this calculator can be used to fill in values of an ice table and find the equilibrium constant of the reaction, initial concentration of reactant, and equilibrium concentration of. Web calculate k c for the following reaction at 300 k where the equilibrium concentrations are [so 3] = 0.400 m, [so. Web it explains how to calculate the equilibrium constant k value given the equilibrium concentrations and equilibrium partial pressures of all reactants and products. Web an ice chart is a means of organizing data when solving a problem for an equilibrium reaction. Make an “ice” table, and enter the known initial concentrations. Acetylene undergoes dimerization to produce vinyl acetylene (butenyne). Next, we need to set up an ice (initial, change, equilibrium) chart for the. Web in this video, i explain how to use an ice chart to calculate the equilibrium concentration of a reversible reaction. Web when finding equilibrium concentrations using an equilibrium constant and initial concentrations, it's often helpful to write out the reaction and construct an ice. Web an ice chart is useful to calculate kc when you have either initial, equilibrium, or a combination of concentrations. It helps in calculating unknown. For starters, we (of course) assume ideal gases (these are both gases at room temperature). Web the calculator uses the initial concentration (i), the change in concentration (c), and the equilibrium concentration (e) to evaluate the state of a chemical equation at equilibrium. This method first lists the concentrations of. Web the equilibrium constant for the following reaction at 600 o c is determined to be kc = 0.495: Web q at 20 o c, k c =3.86 for the reaction below. Web an useful tool in solving equilibrium problems is an ice chart. Web kc = 0.36.
An Ice Chart Is Needed To Calculate Kc If
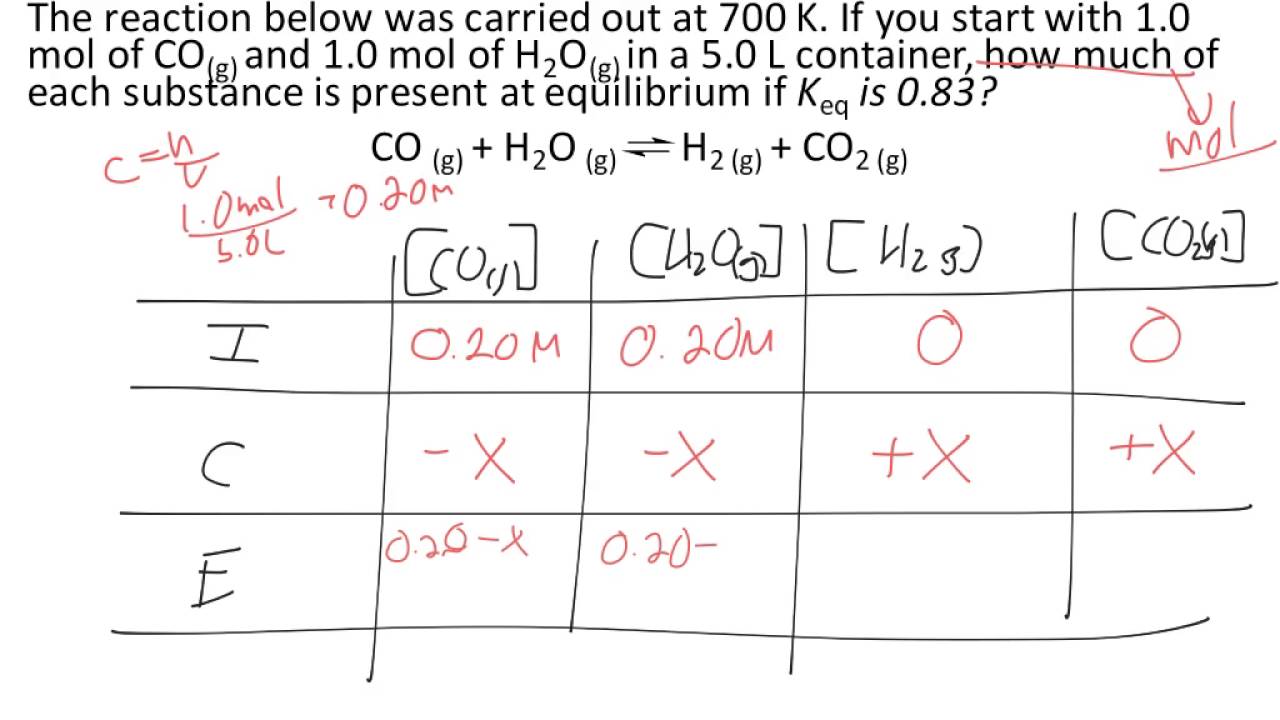
An Ice Chart Is Needed To Calculate Kc If

Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership

Kc calculation (ICE Table) YouTube

An Ice Chart Is Needed To Calculate Kc If

SOLVED Setting up an ICE table to calculate Kc Iodine molecules react
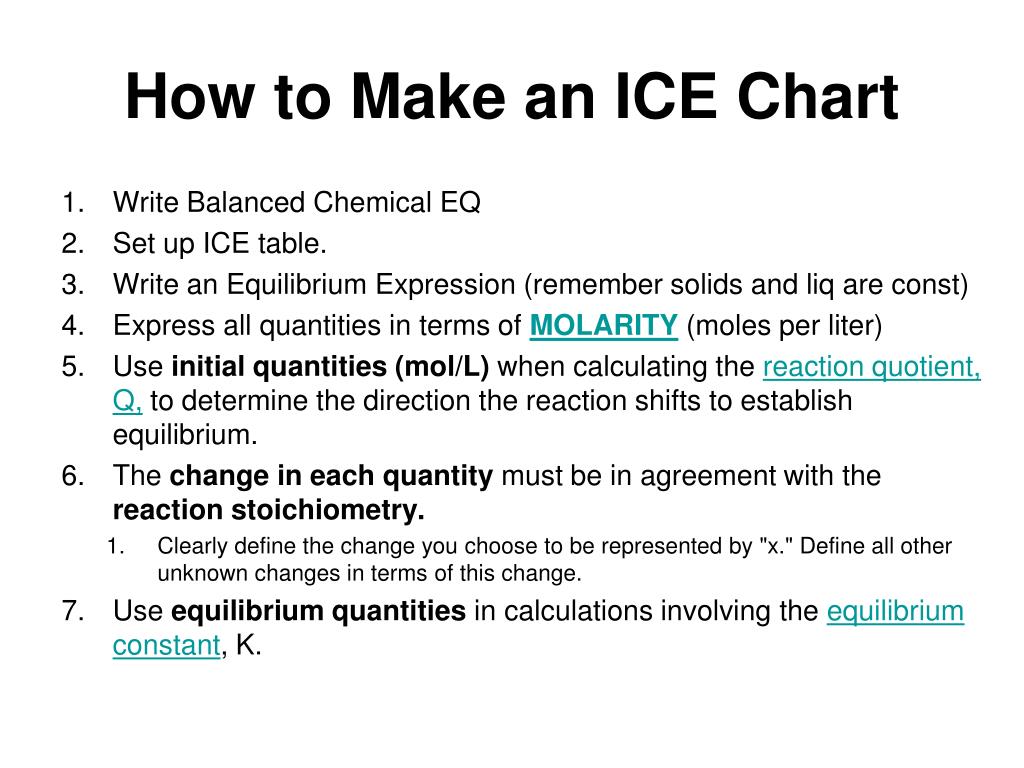
PPT Unit 4 Equilibrium PowerPoint Presentation, free download ID

InitialChangeEquilibrium (ICE) Tables YouTube

Solved An ICE chart is needed to calculate Kc if Select the
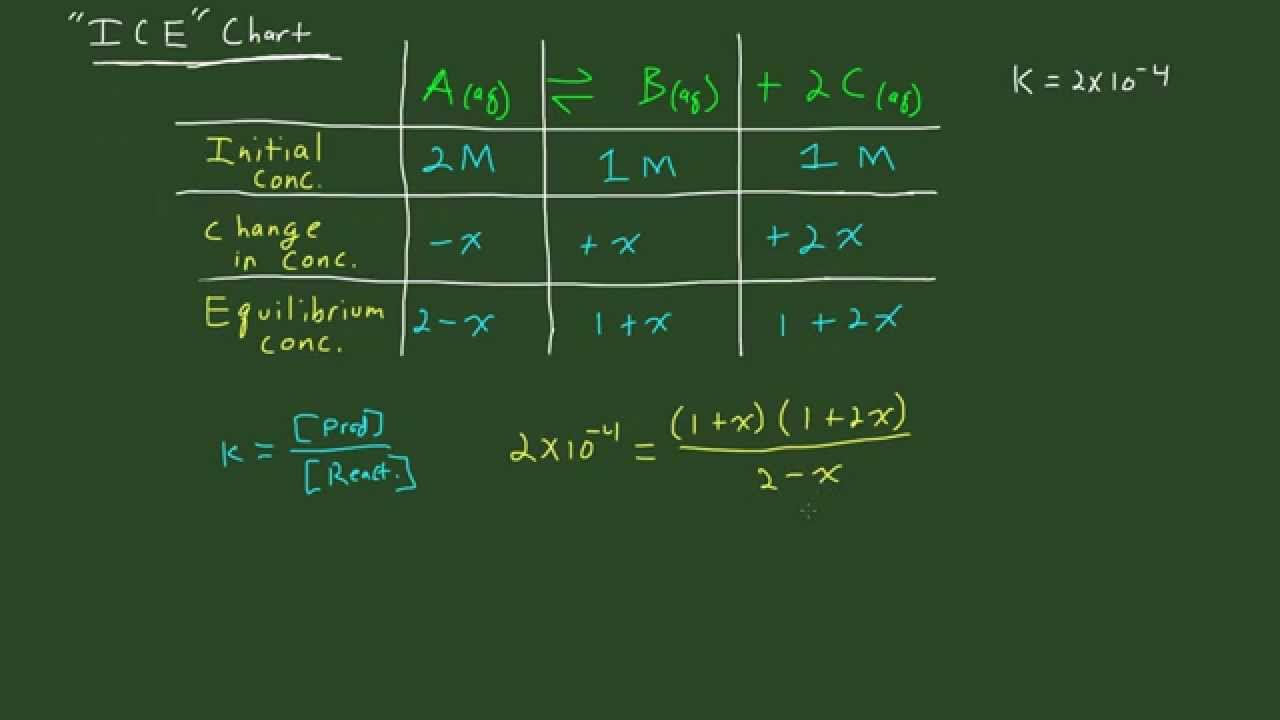
An Ice Chart Is Needed To Calculate Kc If
Web Ice Tables Are Used To Calculate The Value Of Kc If A Combination Of Initial Concentrations And At Lea.view The Full Answer
The Key For Understanding Problem Solving Is:
Web Use An Ice Table To Determine \(K_C\) For The Following Balanced General Reaction:
H 2 O( G ) + Co( G ) ⇆ H 2 ( G ) + Co 2 ( G ) Calculate The Number Of H 2 Moles.
Related Post: