Acid Base Chart
Acid Base Chart - Substances that increase the concentration of h⁺ ions in water.; Web limitations to the arrhenius theory. Table of strong acids the strong acids ionize completely in water to yield or or more protons per acid molecule. Name formula ionization hydrogen iodide or hydroiodic acid hi h+(aq) […] Each of these theories has its uses in chemistry. For each acid, the ionization reaction shows the acid’s conjugate base. Web product details determine at a glance the relative strengths of a host of acids and bases. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. Web this is a list of the strong acids and strong bases. For our purposes, an acid is a substance that increases the concentration of hydrogen ions (h + ) in a solution, usually by donating one of its hydrogen atoms through dissociation.a base, in contrast, raises ph by providing hydroxide (oh − . Use this acids and bases chart to find the relative strength of the most common acids and bases. Web relative strength of acids & bases. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. Web this is a list of the strong acids and strong bases.. Use this acids and bases chart to find the relative strength of the most common acids and bases. Name formula ionization hydrogen iodide or hydroiodic acid hi h+(aq) […] Use this acids and bases chart to find the relative strength of the most common acids and bases. Substances that increase the concentration of h⁺ ions in water.; Web the magnitude. There aren’t very many, so it’s a good idea to memorize them, if you can. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. For each acid, the ionization reaction shows the acid’s conjugate base. Use this acids and bases chart to find the relative strength. Use this acids and bases chart to find the relative strength of the most common acids and bases. Each of these theories has its uses in chemistry. Web product details determine at a glance the relative strengths of a host of acids and bases. Use this acids and bases chart to find the relative strength of the most common acids. Name formula ionization hydrogen iodide or hydroiodic acid hi h+(aq) […] Table of strong acids the strong acids ionize completely in water to yield or or more protons per acid molecule. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The term acid was first used. Use this acids and bases chart to find the relative strength of the most common acids and bases. The acid and base chart is a reference table designed to make determining the strength of. It must be noted, you should always follow lab safety guidelines and never consume or directly touch chemicals.before these regulations were set in stone to protect. For example, the general equation for the ionization of a weak acid in water, where ha is the parent acid and a− is its conjugate base, is as follows: The arrhenius theory has many more limitations than the other two theories. Web relative strength of acids & bases. Web this is a list of the strong acids and strong bases.. However, this does not explain the weak base ammonia (nh 3) which, in the presence of water, releases. Web limitations to the arrhenius theory. There aren’t very many, so it’s a good idea to memorize them, if you can. Substances that increase the concentration of oh⁻ ions in water.; For our purposes, an acid is a substance that increases the. For example, the general equation for the ionization of a weak acid in water, where ha is the parent acid and a− is its conjugate base, is as follows: Substances that increase the concentration of oh⁻ ions in water.; Each of these theories has its uses in chemistry. There aren’t very many, so it’s a good idea to memorize them,. There aren’t very many, so it’s a good idea to memorize them, if you can. Web relative strength of acids & bases. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. However, this does not explain the weak base ammonia (nh 3) which, in the presence. The arrhenius theory has many more limitations than the other two theories. Web limitations to the arrhenius theory. For each acid, the ionization reaction shows the acid’s conjugate base. Table of strong acids the strong acids ionize completely in water to yield or or more protons per acid molecule. Web relative strength of acids & bases. Web the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. However, this does not explain the weak base ammonia (nh 3) which, in the presence of water, releases. There aren’t very many, so it’s a good idea to memorize them, if you can. Web this is a list of the strong acids and strong bases. For our purposes, an acid is a substance that increases the concentration of hydrogen ions (h + ) in a solution, usually by donating one of its hydrogen atoms through dissociation.a base, in contrast, raises ph by providing hydroxide (oh − . The term acid was first used in the seventeenth century; Each of these theories has its uses in chemistry. For example, the general equation for the ionization of a weak acid in water, where ha is the parent acid and a− is its conjugate base, is as follows: Use this acids and bases chart to find the relative strength of the most common acids and bases. Use this acids and bases chart to find the relative strength of the most common acids and bases. Web relative strength of acids & bases.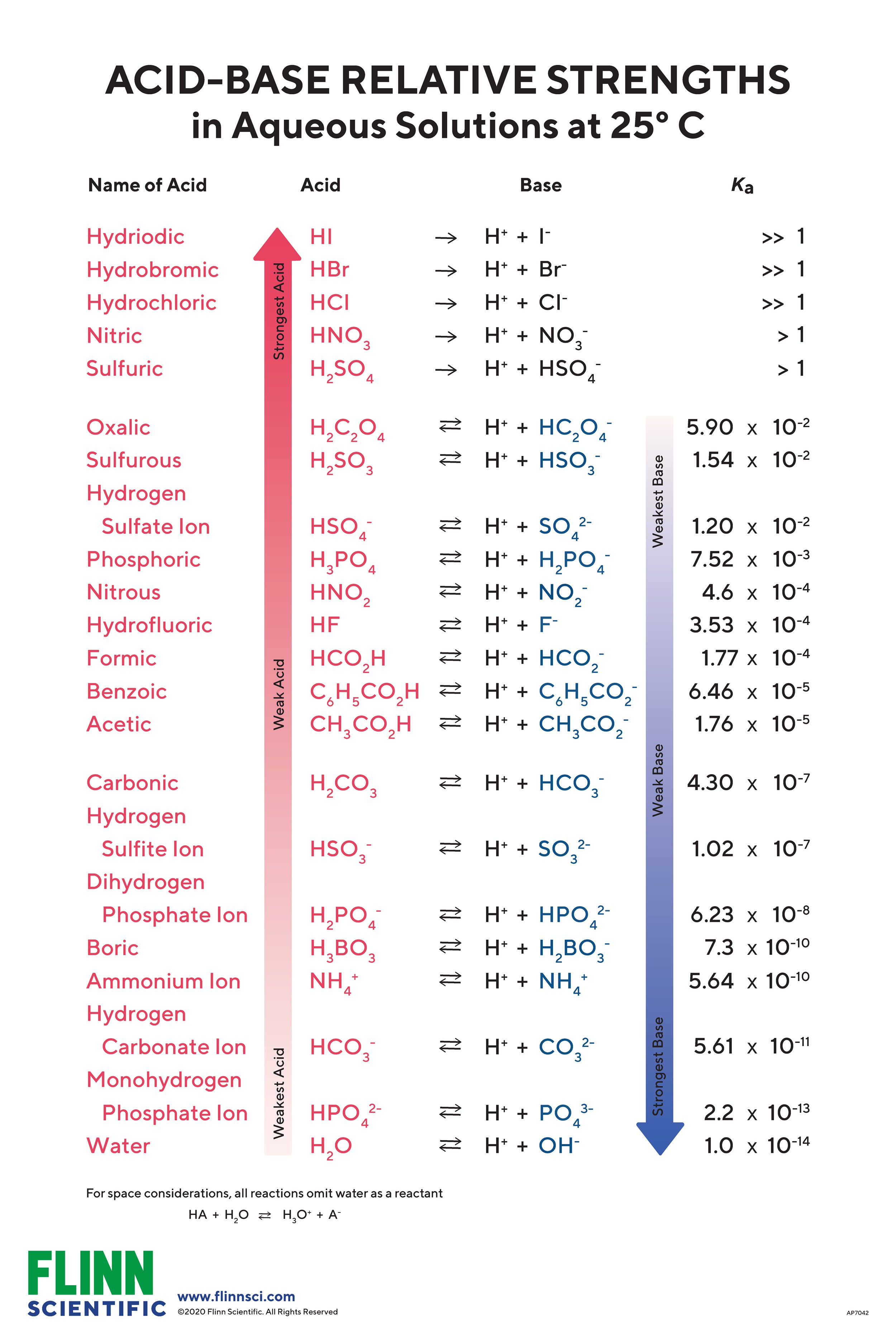
AcidBase Strength Charts for Chemistry

16.5 Strong Acids and Bases Chemistry LibreTexts
What’s The Reaction Between An Acid And A Base?

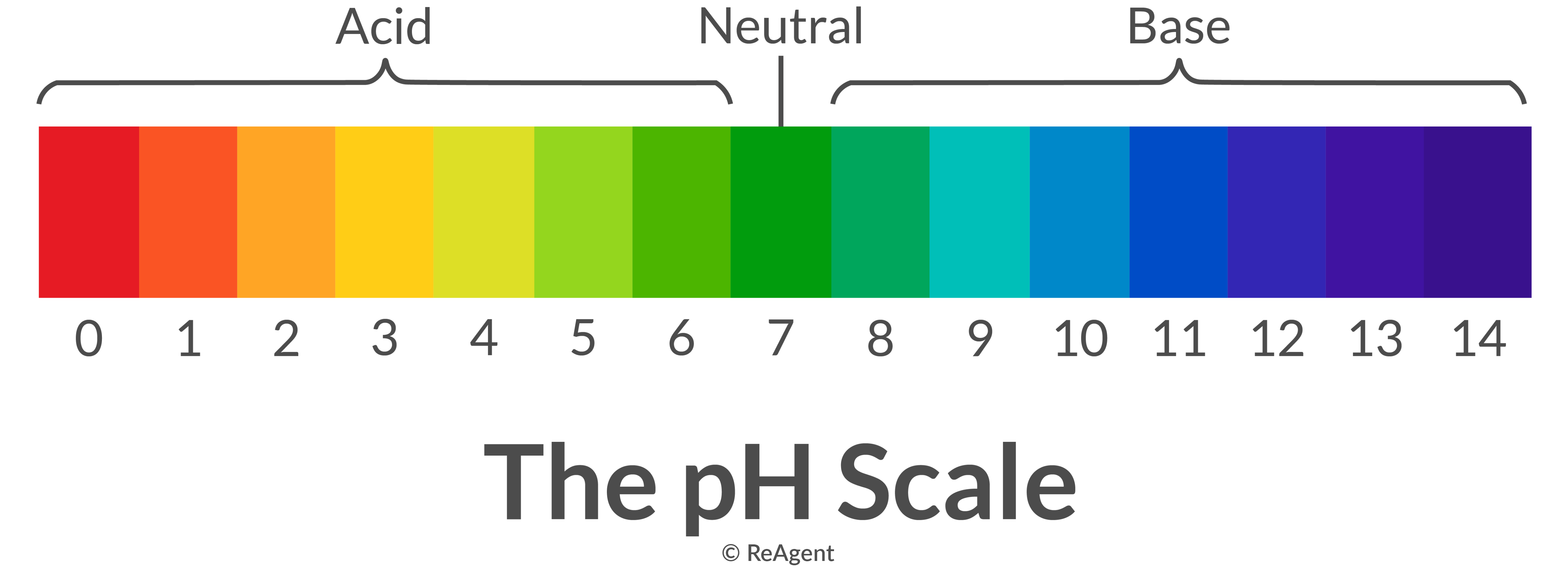
pH Of Acids And Bases Calculate pH Value Chemistry Byju's

Acids and Bases CK12 Foundation

Back to Basics Acids, Bases & the pH Scale Precision Laboratories
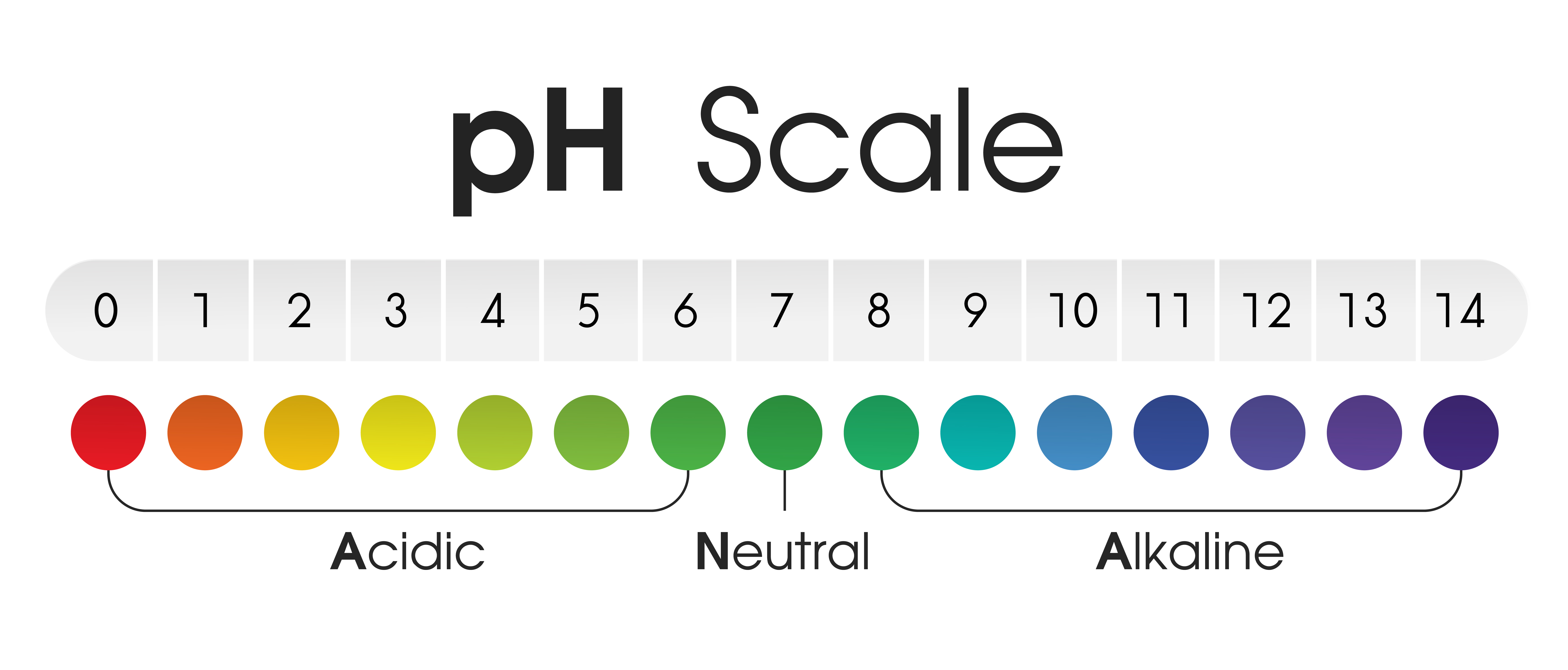
Acid Base Neutral Chart

Interpretation Acid Base Tutorial
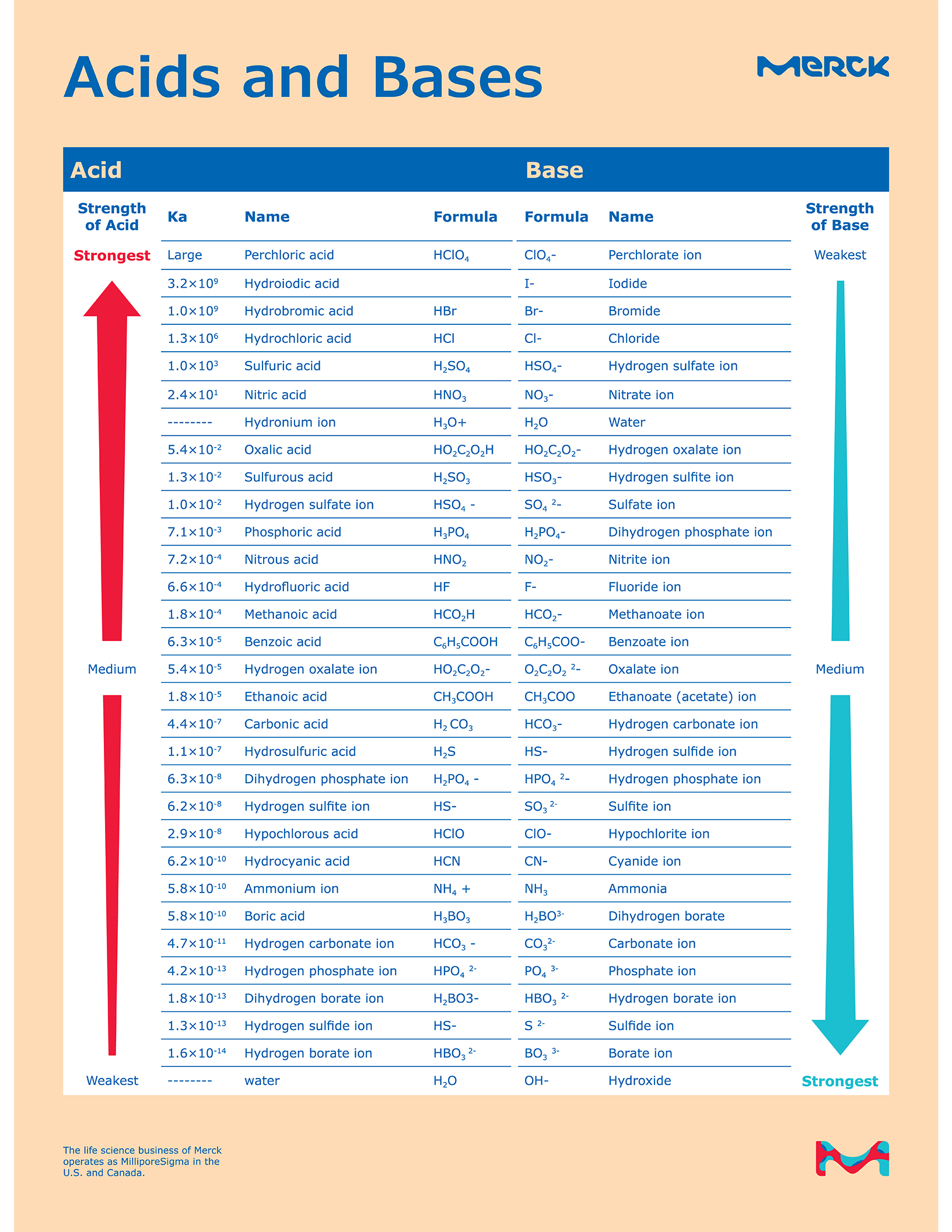
Acid and Base Chart — Table of Acids & Bases
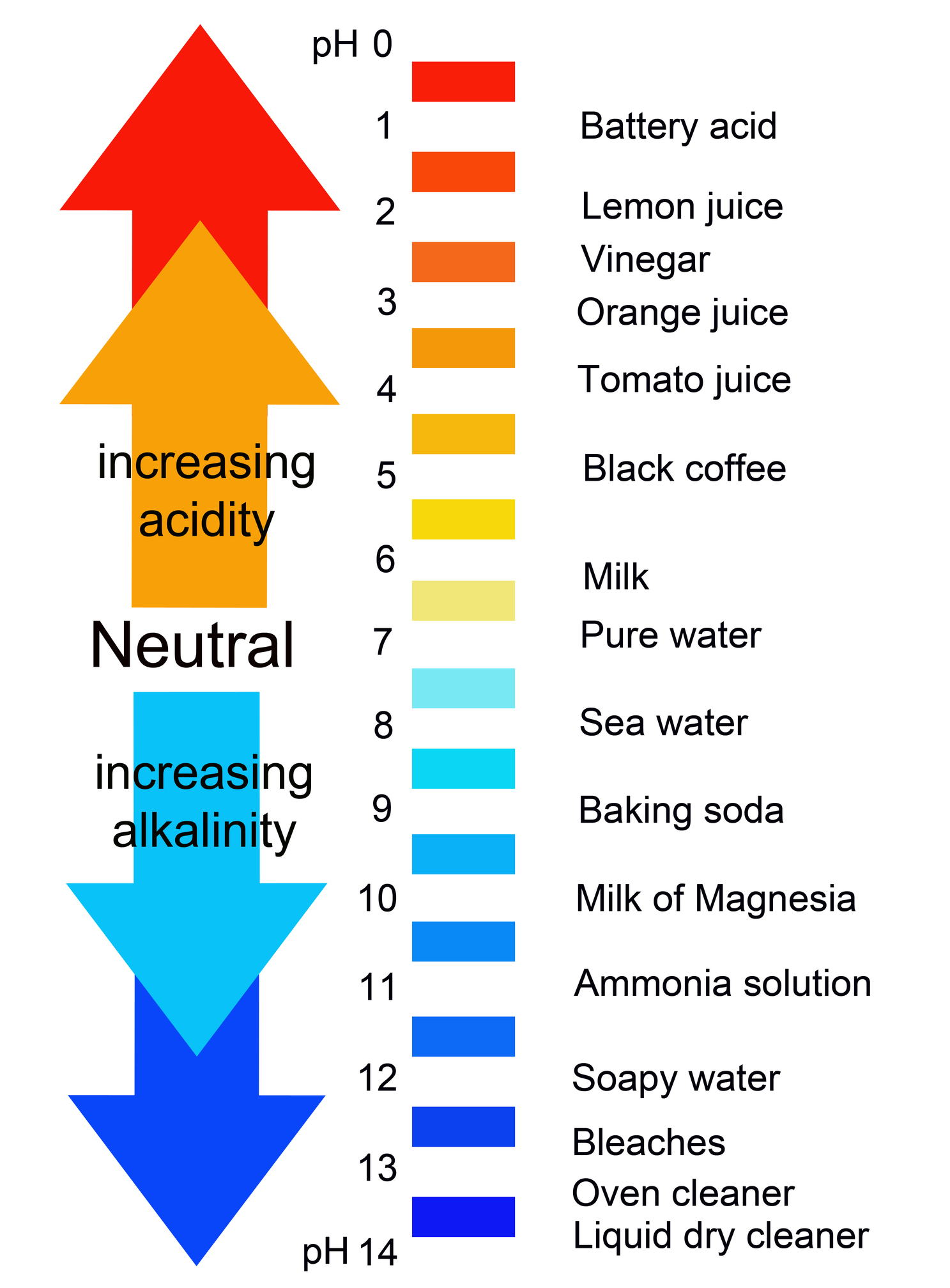
Acids and Bases
The Acid And Base Chart Is A Reference Table Designed To Make Determining The Strength Of.
Substances That Increase The Concentration Of Oh⁻ Ions In Water.;
The Acid And Base Chart Is A Reference Table Designed To Make Determining The Strength Of.
It Must Be Noted, You Should Always Follow Lab Safety Guidelines And Never Consume Or Directly Touch Chemicals.before These Regulations Were Set In Stone To Protect Us, Chemists Used To.
Related Post: