A Study Was Submitted To The Irb Designed
A Study Was Submitted To The Irb Designed - Web an institutional review board (irb), also known as an independent ethics committee (iec), ethical review board (erb), or research ethics board (reb), is a committee at an. Specifically, the plan submitted to an irb for review and to an agency for research support. Web persons intending to carry out research involving human subjects will submit to the irb an application under one of the following four categories: Web the irb recognizes that certain circumstances may warrant approval of research where the study design is not preeminent, but where the risks to participants are virtually non. Web all nih investigators conducting activities that meet the definition of both research and human subject must submit for institutional review board (irb) approval before. The irb approved the study. A decision chart to help guide researchers in making a determination of whether they're conducting human subject research and need to submit an irb. A study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. • the application team list and irb team list. To determine the health utility values (huvs) of overactive bladder (oab) among adults aged ≥65 years and to assess the huv decrements. The irb approved the study. A study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. The irb approved the study and consent form. Web an institutional review board (irb), also known as an independent ethics committee (iec), ethical review board (erb), or research ethics board (reb),. Web therefore, the decision about whether review is required should be made in concert with the irb. Females of 18 years and older. Web all asu and research related projects involving humans as subjects must be reviewed and approved by asu’s institutional review board (irb) prior to. Web we are excited to announce we have an updated study approved by. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. The irb approved the study. • the application team list and irb team list. If you think that your project is limited to evaluative activities and therefore not. Web an institutional review board (irb), also. Web the irb recognizes that certain circumstances may warrant approval of research where the study design is not preeminent, but where the risks to participants are virtually non. The irb approved the study. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Web in. A decision chart to help guide researchers in making a determination of whether they're conducting human subject research and need to submit an irb. Forage field day is a premier regional event designed to bring together the. Web in accordance with the terms of the federalwide assurance (fwa) that columbia university maintains with the federal department of health and human. A study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. The formal design or plan of an experiment or research activity; Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions.. The formal design or plan of an experiment or research activity; • the application team list and irb team list. Web therefore, the decision about whether review is required should be made in concert with the irb. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and. The irb approved the study. Web all asu and research related projects involving humans as subjects must be reviewed and approved by asu’s institutional review board (irb) prior to. The formal design or plan of an experiment or research activity; Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability. Females of 18 years and older. The irb approved the study. A study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Web therefore, the decision about whether review is required should be made in concert with the irb. Web we are excited to announce we have. Web we are excited to announce we have an updated study approved by the irb (#19957)! Females of 18 years and older. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Specifically, the plan submitted to an irb for review and to an agency. When am i required to submit an irb. The irb approved the study and consent form. Web the eirb application includes an option to fill out and complete the required hipaa attestations and submit them to the irb (start a new study in eirb, select hipaa. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Web we are excited to announce we have an updated study approved by the irb (#19957)! Females of 18 years and older. Web a study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Ben beckman, nebraska extension educator. The irb approved the study. Web an institutional review board (irb), also known as an independent ethics committee (iec), ethical review board (erb), or research ethics board (reb), is a committee at an. Web 2024 forage field day moving online. A study was submitted to the irb designed to evaluate the effect of background noise on an individual's ability to concentrate and answer questions. Web persons intending to carry out research involving human subjects will submit to the irb an application under one of the following four categories: Web therefore, the decision about whether review is required should be made in concert with the irb. Forage field day is a premier regional event designed to bring together the. Web in accordance with the terms of the federalwide assurance (fwa) that columbia university maintains with the federal department of health and human services, all research with.
Create & Submit a Study to the IRB Research at Penn State

Create & Submit a Study to the IRB Research at Penn State

New Investigator Toolkit IRB The University of Utah

New Investigator Toolkit IRB The University of Utah
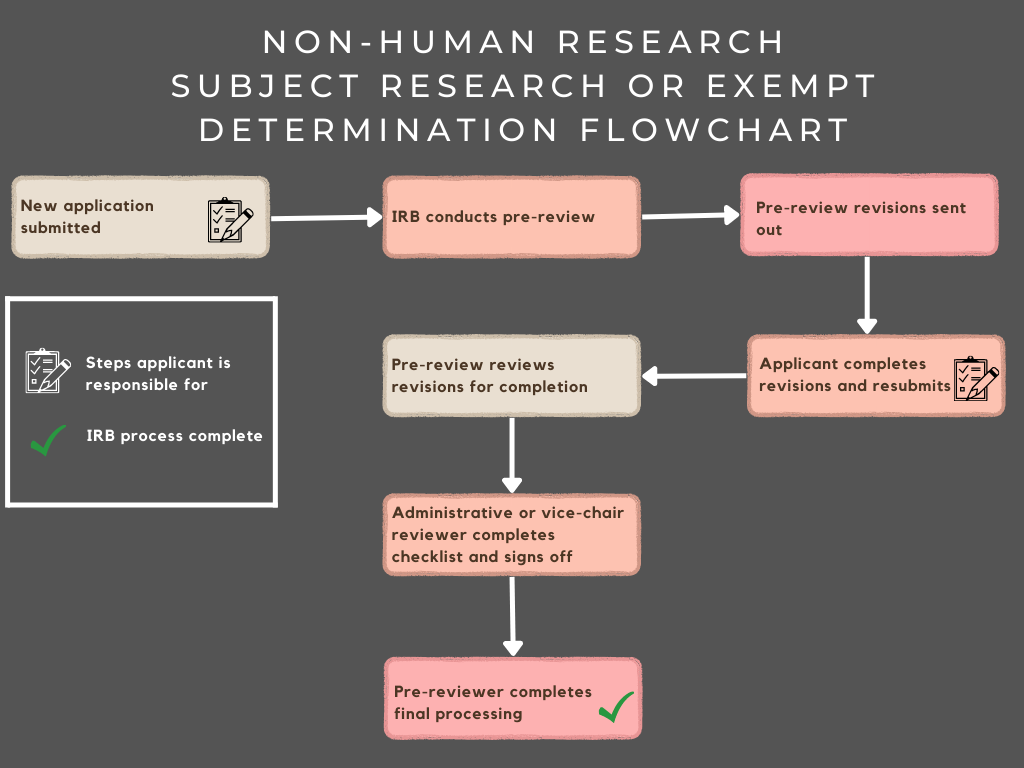
IRB Flow Chart Office of Undergraduate Research
Solved A study was submitted to the IRB designed to evaluate

SOLVED A study was submitted to the IRB designed to evaluate the
Solved A study was submitted to the IRB designed to evaluate
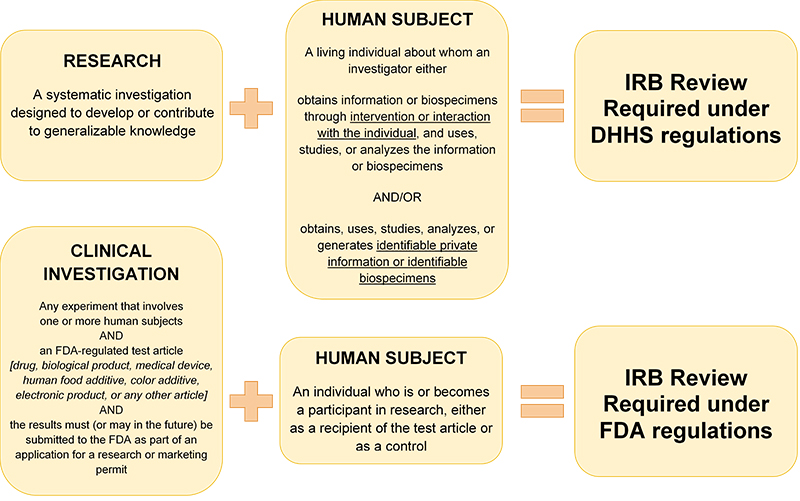
Activities requiring IRB review Virginia Commonwealth University

Study Design IRB By Dr. Manal Thabet YouTube
Web The Irb Recognizes That Certain Circumstances May Warrant Approval Of Research Where The Study Design Is Not Preeminent, But Where The Risks To Participants Are Virtually Non.
Web All Asu And Research Related Projects Involving Humans As Subjects Must Be Reviewed And Approved By Asu’s Institutional Review Board (Irb) Prior To.
Web A Study Was Submitted To The Irb Designed To Evaluate The Effect Of Background Noise On An Individual's Ability To Concentrate And Answer Questions.
To Determine The Health Utility Values (Huvs) Of Overactive Bladder (Oab) Among Adults Aged ≥65 Years And To Assess The Huv Decrements.
Related Post:

